Method Article
Preparation of Intact Tissue for Microscopic Analysis of the Endosperm Cell Layer in Developing and Mature Arabidopsis Seeds
In This Article
Summary
This protocol describes the preparation of intact samples of the endosperm cell layer in Arabidopsis thaliana seeds. The method requires only common laboratory equipment, such as an injection needle and precision forceps, and enables high-resolution fluorescent live-cell imaging of endosperm cells in both developing and mature seeds.
Abstract
In Arabidopsis seeds, the endosperm, a single layer of living cells located between the embryo and the testa, plays a critical role in regulating seed maturation, dormancy, and germination. Microscopic analysis of intact endosperm cells is essential for understanding the physiological functions of the endosperm at cellular and molecular levels. However, sample preparation has been challenging due to the small size of Arabidopsis seeds and the location of the endosperm cell layer beneath the testa. This article details the preparation of intact endosperm cell layer samples suitable for microscopic observation and analysis in both developing and mature seeds. This method enables the observation of large areas and numerous intact endosperm cells without requiring fixation or sectioning. Additionally, the protocol utilizes only standard laboratory equipment, such as injection needles, precision forceps, and stereo microscopes. This approach successfully enables high-resolution live-cell imaging of fluorescent signals, such as green fluorescent protein (GFP), in intact endosperm cells. This method allows for the observation of intracellular localization and movement of various proteins, as well as the morphology of organelles, in the endosperm cells of different Arabidopsis mutants. This protocol contributes to the elucidation of novel endosperm functions and expands the potential for cellular and molecular studies of this essential tissue.
Introduction
Because plants are sessile organisms, seed germination is a crucial event that determines their fate. The decision to germinate is strictly regulated by both internal and environmental factors, such as primary seed dormancy levels, temperature, light intensity and wavelength, and nitrogen concentration1,2,3,4,5,6. Seeds have complex structures consisting of multiple tissue types7. In Arabidopsis dry seeds, the embryo, which develops into a seedling, is surrounded by a single layer of endosperm and the outermost layers, the testa. The testa is composed of multiple layers of dead cells, whereas the embryo and endosperm remain alive even in dry seeds. The endosperm is commonly regarded as a storage tissue that provides nutrients for embryo growth and, together with the testa, confers mechanical resistance to radicle protrusion8,9,10,11,12,13.
Several recent studies have demonstrated that the endosperm plays an essential role in regulating optimal seed germination14,15,16,17. For instance, the photoreceptor phytochrome B (PHYB) in endosperm cells detects either red (R) or far-red (FR) light, regulating germination responses15. The endosperm also functions as a temperature-sensing tissue, suppressing germination responses under high temperatures16. Quality control of the endosperm is critical for optimal seed germination, particularly in long-term stored seeds17.
Live-cell imaging is now necessary to further elucidate the physiological functions of the endosperm. Microscopic analysis of intact endosperm cells expressing fluorescent-tagged proteins allows the investigation of the molecular mechanisms by which the endosperm regulates seed germination. However, preparing intact endosperm cells for microscopic observation is challenging, particularly in Arabidopsis seeds. The seeds are approximately 0.4 mm in diameter, and the endosperm is a single-cell layer located between the embryo and the testa, making precise manipulation difficult. Consequently, despite its important physiological roles, the endosperm has rarely been observed using live-cell imaging.
This article presents a protocol for the rapid preparation of intact endosperm cell layer samples suitable for live-cell imaging in both developing and mature seeds.
Protocol
In this study, two different procedures were established for the preparation of living endosperm cell layer samples: one for developing seeds and one for mature seeds. Slightly different approaches are required depending on the solidity of the testa. The details of the reagents and equipment used are listed in the Table of Materials.
1. Preparation of intact endosperm samples from developing seeds
- Collection of siliques
- Grow Arabidopsis plants on soil or rockwool until blooming.
- Mark fully opened flowers with colored threads (0 days after flowering, 0 DAF).
NOTE: Avoid using green, yellow, or brown threads to mark flowers, as these colors are difficult to distinguish from growing or mature plants and siliques. - Cut off the marked developing siliques at the pedicel (indicated in Figure 1(1)) and collect them in 1.5 mL tubes.
NOTE: Developing siliques from 12-16 DAF are suitable for preparation using this protocol.
- Dissection of developing seeds
NOTE: The following steps must be performed on wet filter paper to protect the samples from desiccation. Manipulations should be done under a stereo microscope.- Split a valve (indicated in Figure 1(2)) from the replum (indicated in Figure 1(1)) using two forceps: one with thick tips for holding and the other with sharp tips for tearing).
- Gently pick up developing seeds from the siliques using forceps with the tips closed to avoid damaging the seeds (Figure 1(2)).
- Holding the seed with forceps without crashing the seed, make a scar approximately 0.2 mm in size on the testa and endosperm surrounding the embryo using an injection needle (27 G, 0.40 mm × 19 mm) (Figure 1(3)).
NOTE: The optimal location for making the scar is at the junction of the cotyledons and radicle. - Push out the embryo by pinching the seed with forceps (Figure 1(4)). Do not crush the empty seed envelope, which consists of the testa and endosperm, and try to maintain its round shape.
- Insert the injection needle into the empty seed envelope at the scar, piercing it from the inside out (Figure 1(5)).
- Keep the needle in position, scratch the surface of the testa using forceps with the tips closed, and cut one side of the empty seed envelope to allow it to open (Figure 1(6)).
- Open the empty seed envelope into a sheet using forceps with sharp tips (Figure 1(7)). The sample should now be isolated as a bilayer sheet consisting of the endosperm and testa layers (Figure 1(8)).
NOTE: If the sample tends to curl up when water is used as the mounting medium in step 3 below, divide the sheet-form sample into two pieces. Seeds harvested from siliques at around 18 DAF (at this stage, the testa is brown but not yet completely dry) can also be processed using this protocol, although the seeds must be imbibed for several minutes before preparation.
2. Preparation of intact endosperm samples from mature seeds
- Imbibition of mature seeds
- Add 1 mL of double-distilled water to a 1.5 mL tube containing dry Arabidopsis seeds.
- Keep the seeds imbibed for at least 40 min at room temperature (Figure 2(1)).
NOTE: Dry seeds and seeds within 40 min of imbibition are difficult to scar at the testa and endosperm and to remove the embryo from inside the seed without damaging the empty seed envelope in steps 2.2.1 and 2.2.2, whereas a longer imbibition time facilitates manipulation. - Use a 1000 µL micropipette to transfer the imbibed seeds onto wet filter paper.
- Dissection of mature seeds
NOTE: The following steps must be performed on wet filter paper to protect the samples from desiccation. Manipulations should be conducted under a stereo microscope.- Holding the seed with forceps without crashing the seed, make a scar approximately 0.2 mm in size on the testa and endosperm surrounding the embryo using an injection needle (Figure 2(2)).
- Push out the embryo by pinching the seed with forceps (Figure 2(3)). Do not crush the empty seed envelope, which includes the testa and endosperm. Try to maintain its round shape.
- Cut the upper and lower sides of the empty seed envelope with an injection needle to shape it into a cylinder (Figure 2(4)).
- Cut the cylindrical-shaped empty seed envelope along its central axis to separate it into two pieces (Figure 2(5)). The samples should be isolated as bilayer sheets consisting of the endosperm layer and the testa layer (Figure 2(6)).
3. Microscopic observation
- Place the endosperm samples in sheet form on a glass slide and mount them in water or perfluorodecalin (PFD).
NOTE: If air bubbles remain between the sample and the coverslip, perfluorodecalin (PFD), which has been reported to be particularly useful for imaging samples containing air pockets, such as leaves18,19, would be useful. PFD is known to have the lowest surface tension, allowing it to easily fill the air spaces on the sample surface. For time-lapse imaging, however, the use of water as a mounting medium is recommended, as the water content in mature seeds should be abundant to maintain cellular liquidity. - Place a coverslip gently over the sample. Ensure that the endosperm layer is facing the coverslip.
NOTE: Nail polish or grease can be used to seal the edges of the coverslip to prevent desiccation of the sample and the mounting medium.
Results
Using the protocol shown in Figure 1, endosperm samples were prepared from developing seeds harvested from siliques at 14 DAF (at this stage, the testa is still green). Numerous endosperm cells across a large area and their intracellular structures were observed (Figure 3A). In this experiment, seeds expressing PHYB fused with GFP at the C-terminus (PHYB-GFP) were used. It is well known that PHYB translocates to the nucleus upon activation by red light and forms PHYB-positive speckles, known as photobodies (PBs), which are involved in seed germination4,20,21,22. The samples were harvested from plants grown under continuous white light conditions at 22 °C. As shown in Figure 3B, PBs within the nucleus were detected in endosperm cells. This result corresponds to a previous study showing that PHYB in the endosperm regulates optimal seed germination15. Additionally, live-cell time-lapse imaging was conducted to confirm that the isolated endosperm cells retained biological activity even after sample preparation by observing phytochrome FR/R photoreversibility (Figure 3C). Endosperm samples were prepared from developing seeds harvested from siliques at around 17-18 DAF. PBs were detected within the nucleus upon dissection, whereas they completely disappeared after irradiation with far-red light. Subsequently, red light irradiation again induced PBs. This phytochrome photoreversibility was detected twice, indicating that the endosperm cells isolated by this protocol retained biological activity for at least 4.5 h after dissection (Figure 3C).
Next, endosperm samples from mature seeds (where the testa is brown) were prepared, as shown in Figure 2. In this experiment, the motility of mitochondria was observed using seeds expressing mitochondrial-targeted GFP (mtGFP)23, which had been stored in a desiccator at room temperature in the dark for approximately one year after harvesting. It has been reported that mitochondria in the embryo actively move and dynamically change morphology after imbibition and transfer to germination conditions24. Fluorescent time-lapse imaging of mitochondria in intact endosperm cells after 3 h of seed imbibition was performed, and mitochondrial movement was detected (Supplementary Movie 1). Mitochondria in the endosperm moved immediately within the cytosol after a short period of imbibition at 22 °C. After 1 day of seed imbibition at 22 °C, more dynamically moving mitochondria were observed (Figure 4 and Supplementary Movie 2).
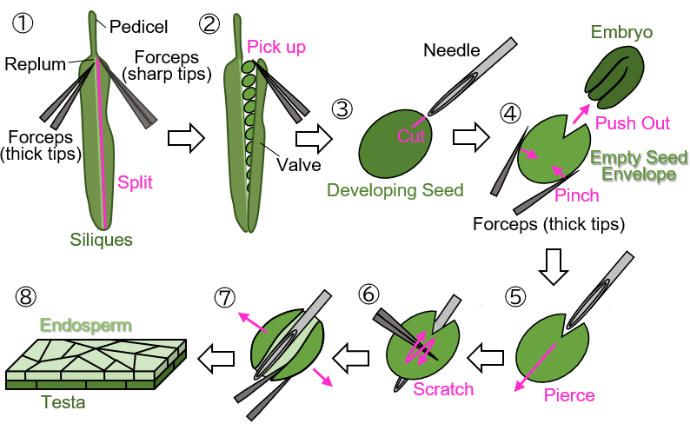
Figure 1: Endosperm sample preparation from developing seeds. Step 1: A valve is split from the replum. Step 2: The developing seeds are collected. Step 3: A scar is created on the testa and endosperm using an injection needle. Step 4: The embryo is removed by pinching the seed with forceps. Step 5: The injection needle is inserted through the empty seed envelope from the inside to the outside. Step 6: The surface of the testa is scratched using forceps. Step 7: The empty seed envelope is opened into a sheet form using forceps. Step 8: The final endosperm sheet sample is prepared for microscopic observation. All steps are performed on wet filter paper under a stereo microscope. Please click here to view a larger version of this figure.

Figure 2: Endosperm sample preparation from mature seeds. Step 1: Dry seeds are imbibed in water for more than 40 min in a 1.5 mL tube. Step 2: A scar is created on the testa and endosperm using an injection needle. Step 3: The embryo is removed by pinching the seed with forceps. Step 4: The upper and lower sides of the empty seed envelope are cut to shape it into a cylinder. Step 5: The cylindrical-shaped empty seed envelope is cut along its central axis. Step 6: The final endosperm sheet sample is prepared for microscopic observation. All steps are performed on wet filter paper under a stereo microscope. Please click here to view a larger version of this figure.

Figure 3: Fluorescent imaging of an intact endosperm cell layer using developing seeds. Intact endosperm cell layer samples were prepared from developing seeds of 14-day-after-flowering (DAF) siliques (A,B) and 17-18 DAF siliques (C). Microscopic imaging was performed using a confocal laser scanning microscope equipped with a Plan-Apochromat 63× oil-immersion objective lens and a white-light laser. (A) A confocal image of the endosperm under differential interference contrast (DIC) shows large areas with numerous intact endosperm cells. Scale bar: 20 µm. (B) Confocal images of the endosperm expressing PHYB-GFP are shown, with PHYB-GFP in green and chlorophyll autofluorescence in cyan. Photobodies (PBs) in a nucleus are indicated by white arrowheads. Scale bars: 10 µm; enlarged image scale bar: 2.5 µm. The GFP signal was separated from the autofluorescence of protein storage vacuoles (PSVs) using fluorescence-lifetime microscopy (FLIM). Fluorescence lifetimes were obtained using TauScan and separated using the TauSeparation function. The average fluorescence lifetime for GFP was 2.2 ns. GFP fluorescence was excited at 488 nm and collected between 500-530 nm, while chlorophyll autofluorescence was excited at 405 nm and collected between 688-729 nm. (C) Confocal images of the endosperm expressing PHYB-GFP under continuous white light (cWL), continuous far-red light (cFR), or continuous red light (cR) are presented. PHYB-GFP is shown in green, and PSV autofluorescence is shown in magenta. Scale bars: 10 µm; enlarged image scale bar: 2.5 µm. Light intensities: WL, 46.5 µmol/m2/s; FR, 35.2 µmol/m2/s; R, 18.6 µmol/m2/s. PSV autofluorescence was excited at 561 nm and collected between 565-621 nm. The GFP signal was separated using the same method described above. Please click here to view a larger version of this figure.
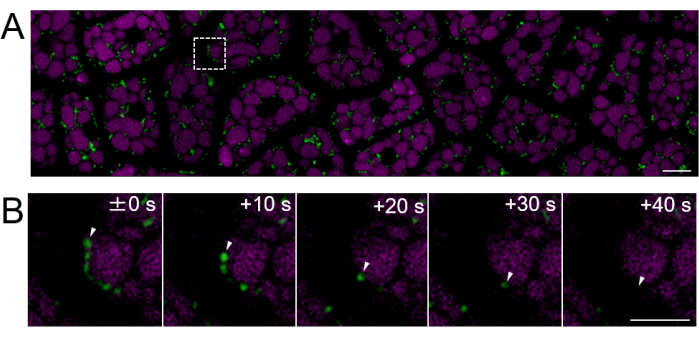
Figure 4: Fluorescent live-cell imaging of an intact endosperm cell layer using mature seeds. Microscopic imaging was performed using the same method described in Figure 3. (A) A confocal image of the entire intact endosperm cell layer, obtained from Supplementary Movie 2, is shown. Mitochondria are labeled in green, and PSV autofluorescence is labeled in magenta. Scale bar: 10 µm. (B) A time-lapse series of enlarged images from the dashed-line square area in panel (A) shows the movement of a mitochondrion, indicated by white arrowheads. Scale bar: 5 µm. Please click here to view a larger version of this figure.
Supplementary Movie 1: A time-lapse video of mtGFP in the endosperm cell layer of a mature seed 3 h after imbibition is shown. One frame was taken every 10 s, and the frame rate of the video is 10 frames/s. Scale bar: 10 µm. Please click here to download this File.
Supplementary Movie 2: A time-lapse video of mtGFP in the endosperm cell layer of a mature seed 1 day after imbibition is shown. One frame was taken every 10 s, and the frame rate of the video is 10 frames/s. Scale bar: 10 µm. Please click here to download this File.
Discussion
Roles of the endosperm in seed germination have been revealed through genetic and biochemical analyses using separated seed tissues, such as gene expression analysis and the quantification of lipids and phytohormones9,14,25,26,27. An in vitro seed coat bedding assay, combining the empty seed envelope (endosperm and testa) with an embryo isolated from different Arabidopsis mutants, uncovered molecular mechanisms underlying germination processes14,15,16,28. However, sample preparation for microscopic observation has been challenging due to the small size of Arabidopsis seeds and the location of the endosperm cell layer beneath the testa. In particular, observation of the endosperm cell layer during seed development has rarely been conducted without chemical fixation and sectioning.
During sample preparation, the empty seed envelope from developing seeds is fragile because the testa is living and thus soft. Here, two protocols were established for the rapid preparation of intact endosperm cell layer samples suitable for microscopic observation and analysis in developing seeds without requiring fixation or sectioning. In the case of sample preparation from mature seeds, the testa is dead and sturdy, causing the empty seed envelope to retain its curvature when mounted on a slide glass. This curvature makes it difficult to observe the endosperm cell layer clearly across a wide range of focus. Therefore, the dissection method for developing seeds was slightly modified to appropriately trim the empty seed envelope. By separating it into two pieces using this method, clear observation of numerous endosperm cells while maintaining focus was achieved.
Various imaging reagents (for staining DNA, reactive oxygen species, acidic compartments, etc.) have been developed, and some have been applied to Arabidopsis plants29,30,31,32. Endosperm cell layer samples prepared using this protocol have already been utilized for microscopic observation with LysoTracker and Propidium Iodide staining, contributing to the evaluation of endosperm function17. Since the discovery of GFP in 196233, GFP-tagged proteins have become essential tools for elucidating the dynamics of intracellular structures23,24,34,35,36,37,38. By preparing endosperm samples according to this protocol and employing the aforementioned methods, it is possible to perform live-cell imaging of the endosperm during seed development and germination, as shown in Figure 3 and Figure 4, thereby elucidating intracellular events and the localization of specific proteins. Furthermore, the application of specific inhibitors (such as protease inhibitors, protein modification enzyme inhibitors, and inhibitors of cellular dynamics)39,40,41,42 to live endosperm cells using this protocol is expected to provide deeper insights into the intracellular events and molecular mechanisms underlying endosperm function during seed development and germination.
One limitation of this protocol is that endosperm cell layers from completely dried seeds cannot be prepared because it is difficult to separate the seeds into the embryo and the empty seed envelope. As shown in Figure 2, an imbibition time of at least 40 min is necessary to separate mature seeds. It is important to note that several cellular events within the endosperm cells may be induced upon imbibition. Another limitation is that this protocol does not allow for the observation of endosperm tissue during the early stages of seed development, particularly prior to 10 DAF, as it is specifically designed for observing the single-cell endosperm layer. During these early stages, the tissue preceding the formation of the single-cell endosperm layer-a non-layered endosperm-fills the interior of developing seeds43, making it difficult to prepare endosperm samples.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgements
We thank Drs. Matsushita and Oka of Kyoto University for providing the phyB mutant expressing PHYB-GFP driven by the 35S promoter. This study was partly supported by a Grant-in-Aid for Scientific Research on Innovative Areas, Research in a Proposed Research Area (19H05713 to K.Y.).
Materials
| Name | Company | Catalog Number | Comments |
| 1.5 mL Microcentrifuge Tubes | Watoson Bio Lab | 131-815C | |
| Coverslip (18 x 18 mm) | Matsunami Glass Ind.,Ltd. | C218181 | |
| DDW | Water for mountting | ||
| Filter Paper No.526 (400 x 400 mm) | ADVANTEC VIETNAM CO., LTD. | 02453400 | |
| Genki-kun Seru Senyo yodo kopu N-150 (55 L) | Katakura & Co-op Agri Corporation | Soils for Plant Growth | |
| Glass slide (26mm x 76 mm) | Matsunami Glass Ind.,Ltd. | S1215 | |
| Grodan AO 36 x 36 x 40 mm Cubes | Grodan | Rockwools for Plant Growth | |
| Iris Scissors | Premium Plus Japan Co.,Ltd. | FC-0212 | |
| Jewelers forceps, Dumont No. 5 (4 1/4 in.) | Dumont | F6521 | Forceps for Tearing |
| Leica Application Suite X (LAS X) | Leica | Software for Sterallis 8 | |
| Leica Microsystems Immersion Oil for Microscopes | Very Low Autofluorescence Immersion Oil | THMOIL-10LF | |
| LIOR precision forceps 110mm SL-14 | KENIS Ltd. | KN33450438 | Forceps for Holding |
| NAIL HOLIC | KOSE | Nail polish | |
| Needls 27G 3/4 (19 mm) RB | Misawa Medical Industry Co., Ltd. | A Ingection Needle for Cutting | |
| Nichipet Air 1000 uL | Nichiryo | 00-NAR-1000 | A 1000 µL Micropipette |
| Perfluorodecalin | APOLLO SCIENTIFIC | PC5960 | Reagents for mounting |
| Red light/far-red light LED panel | TOKYO RIKAKIKAI CO., LTD. | 10147599 | |
| Schappe Spun #60 | Fujix Co., Ltd. | Thread | |
| SPINKOTE Lubricant 2 oz | BECKMAN COULTER | 306812 | Grease |
| Sterallis 8 | Leica | Confocal Laser Scanning Microscopy | |
| Stereomicroscope Stemi 305 cam W | Carl Zeiss NTS Ltd. | 491903-0017-000 | |
| White light LED | PANASONIC | FL40SSW/37 |
References
- Bentsink, L., Jowett, J., Hanhart, J. C., Koornneef, M. Cloning of DOG1, a quantitative trait locus controlling seed dormancy in Arabidopsis. Proc Natl Acad Sci USA. 103 (45), 17042-17047 (2006).
- Toh, S., et al. High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiol. 146 (3), 1368-1385 (2008).
- Seo, M., Nambara, E., Choi, G., Yamaguchi, S. Interaction of light and hormone signals in germinating seeds. Plant Mol Biol. 69 (4), 463-472 (2009).
- Shinomura, T., et al. Action spectra for phytochrome A- and B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc Natl Acad Sci USA. 93 (15), 8129-8133 (1996).
- Liu, Y., et al. Nitric oxide-induced rapid decrease of abscisic acid concentration is required in breaking seed dormancy in Arabidopsis. New Physiol. 183 (4), 1030-1042 (2009).
- Yan, D., et al. NIN-like protein 8 is a master regulator of nitrate-promoted seed germination in Arabidopsis. Nat Commun. 7, 13179 (2016).
- Yan, D., Duermeyer, L., Leoveanu, C., Nambara, E. The functions of the endosperm during seed germination. Plant Cell Physiol. 55 (9), 1521-1533 (2014).
- Lopes, A. M., Larkins, A. B. Endosperm origin, development, and function. Plant Cell. 5 (10), 1383-1399 (1993).
- Penfield, S., et al. Reserve mobilization in the Arabidopsis endosperm fuels hypocotyl elongation in the dark, is independent of abscisic acid,and requires PHOSPHOENOLPYRUVATE CARBOXYKINASE1. Plant Cell. 16 (10), 2705-2718 (2004).
- Leubner-Metzger, G., Fründt, C., Meins, F. Effects of gibberellins, darkness and osmotica on endosperm rupture and class I β-1,3-glucanase induction in tobacco seed germination. Planta. 199, 282-288 (1996).
- Sargant, E. Recent work on the results of fertilization in angiosperms. Ann Bot. 14 (4), 689-712 (1900).
- Groot, P. S., Karssen, M. C. Gibberellins regulate seed germination in tomato by endosperm weakening: A study with gibberellin-deficient mutants. Planta. 171 (4), 525-531 (1987).
- Groot, P. S., Kieliszewa-Rokicka, B., Vermeer, E., Karssen, M. C. Gibberellin-induced hydrolysis of endosperm cell walls in gibberellin-deficient tomato seeds prior to radicle protrusion. Planta. 174, 500-504 (1988).
- Lee, P. K., Piskurewicz, U., Turečková, V., Strnad, M., Lopez-Molina, L. A seed coat bedding assay shows that RGL2-dependent release of abscisic acid by the endosperm controls embryo growth in Arabidopsis dormant seeds. Proc Natl Acad Sci USA. 107 (44), 19108-19113 (2010).
- Lee, P. K., et al. Spatially and genetically distinct control of seed germination by phytochromes A and B. Genes Dev. 26 (17), 1984-1996 (2012).
- Piskurewicz, U., Sentandreu, M., Iwasaki, M., Glauser, G., Lopez-Molina, L. The Arabidopsis endosperm is a temperature-sensing tissue that implements seed thermoinhibition through phyB. Nat Commun. 14, 1202 (2023).
- Shinozaki, D., Takayama, E., Kawakami, N., Yoshimoto, K. Autophagy maintains endosperm quality during seed storage to preserve germination ability in Arabidopsis. Proc Natl Acad Sci USA. 121 (14), e2321612121 (2024).
- Littlejohn, R. G., Gouveia, D. J., Edner, C., Smirnoff, N., Love, J. Perfluorodecalin enhances in vivo confocal microscopy resolution of Arabidopsis thaliana mesophyll. New Phytol. 186 (4), 1018-1025 (2010).
- Littlejohn, R. G., Love, J. A simple method for imaging Arabidopsis leaves using perfluorodecalin as an infiltrative imaging medium. J Vis Exp. (59), e3394 (2012).
- Chen, M., Schwab, R., Chory, J. Characterization of the requirements for localization of phytochrome B to nuclear bodies. Proc Natl Acad Sci USA. 100 (24), 14493-14498 (2003).
- Matsushita, T., Mochizuki, N., Nagatani, A. Dimers of the N-terminal domain of phytochrome B are functional in the nucleus. Nature. 424 (6948), 571-574 (2003).
- Buskirk, V. K. E., Decker, V. P., Chen, M. Photobodies in light signaling. Plant Physiol. 158 (1), 52-60 (2012).
- Logan, C. D., Leaver, J. C. Mitochondria-targeted GFP highlights the heterogeneity of mitochondrial shape, size and movement within living plant cells. J Exp Bot. 51 (346), 865-871 (2000).
- Paszkiewicz, G., Gualberto, M. J., Benamar, A., Macherel, D., Logan, C. D. Arabidopsis seed mitochondria are bioenergetically active immediately upon imbibition and specialize via biogenesis in preparation for autotrophic growth. Plant Cell. 29 (1), 109-128 (2017).
- Lefebvre, V., et al. Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J. 45 (3), 309-319 (2006).
- Okamoto, M., et al. CYP707A1 and CYP707A2, which encode abscisic acid 8' hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol. 141 (1), 97-107 (2006).
- Endo, A., et al. Tissue-specific transcriptome analysis reveals cell wall metabolism, flavonol biosynthesis and defense responses are activated in the endosperm of germinating Arabidopsis thaliana seeds. Plant Cell Physiol. 53 (1), 16-27 (2012).
- Lee, P. K., Lopez-Molina, L. A seed coat bedding assay to genetically explore in vitro how the endosperm controls seed germination in Arabidopsis thaliana. J Vis Exp. (81), e50732 (2013).
- Uno, K., Sugimoto, N., Sato, Y. N-aryl pyrido cyanine derivatives are nuclear and organelle DNA markers for two-photon and super-resolution imaging. Nat Commun. 12, 2650 (2021).
- Shinozaki, D., et al. Autophagy increases zinc bioavailability to avoid light-mediated reactive oxygen species production under zinc deficiency. Plant Physiol. 182 (3), 1284-1296 (2020).
- Laxmi, A., Pan, J., Morsy, M., Chen, R. Light plays an essential role in intracellular distribution of auxin efflux carrier PIN2 in Arabidopsis thaliana. PLOS One. 3 (1), e1510 (2008).
- Rigal, A., Doyle, M. S., Robert, S. Live cell imaging of FM4-64, a tool for tracing the endocytic pathways in Arabidopsis root cells. Methods Mol Biol. 1242, 93-103 (2015).
- Shimomura, O., Johnson, H. F., Saiga, Y. Extraction, purification and properties of Aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 59 (3), 223-239 (1962).
- Holzinger, A., Buchner, O., Lütz, C., Hanson, R. M. Temperature-sensitive formation of chloroplast protrusions and stromules in mesophyll cells of Arabidopsis thaliana. Protoplasma. 230, 23-30 (2007).
- Mano, S., et al. Distribution and characterization of peroxisomes in Arabidopsis by visualization with GFP: dynamic morphology and actin-dependent movement. Plant Cell Physiol. 43 (3), 331-341 (2002).
- Nelson, K. B., Cai, X., Nebenführ, A. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 51 (6), 1126-1136 (2007).
- Goto, C., Tamura, K., Fukao, Y., Shimada, T., Hara-Nishimura, I. The novel nuclear envelope protein KAKU4 modulates nuclear morphology in Arabidopsis. Plant Cell. 26 (5), 2143-2155 (2014).
- Geldner, N., et al. combinatorial analysis of membrane compartments in intact plants with a multicolor marker set. Plant J. 59 (1), 169-178 (2009).
- Jang, I. -. C., Henriques, R., Seo, S. H., Nagatani, A., Chua, N. -. H. Arabidopsis phytochrome interacting factor proteins promote phytochrome B polyubiquitination by COP1 E3 ligase in the nucleus. Plant Cell. 22 (7), 2370-2383 (2010).
- AI-Sady, B., Ni, W., Kircher, S., Schäfer, E., Quail, H. P. Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol Cell. 23 (4), 439-446 (2006).
- Lam, K. S., et al. BFA-induced compartments from the Golgi apparatus and trans-Golgi network/early endosome are distinct in plant cells. Plant J. 60 (5), 865-881 (2009).
- Yoshimoto, K., et al. Processing of ATG8s, ubiquitin-like proteins, and their deconjugation by ATG4s are essential for plant autophagy. Plant Cell. 16 (11), 2967-2983 (2004).
- Xu, G., Zhang, X. Mechanisms controlling seed size by early endosperm development. Seed Biol. 2, 1 (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved