Method Article
Widespread Transduction of Mouse Neocortical Neurons by Subarachnoid Injection of AAV2
In This Article
Summary
A new technique for widespread delivery of adeno-associated virus that uses subarachnoid virus infusion is described. This method not only ensures widespread transduction of mouse neocortical neurons in superficial layers but also results in selective expression of the transgene in layer five pyramidal neurons, even when using a non-selective promoter.
Abstract
Recombinant adeno-associated viruses are a flexible and powerful tool for the delivery and expression of various genes of interest in many areas of experimental biology, particularly in neuroscience. The most popular method to drive the expression of a desired transgene in a particular brain area is to inject an AAV vector directly into the brain parenchyma. However, this method does not allow widespread neuronal transduction that is required for some in vivo experiments. In this article, we present a new technique for widespread gene expression in the mouse neocortex based on viral infusion into the subarachnoid space of the brain. This neuronal labeling method not only ensures widespread transduction of neurons in adult mouse superficial neocortical layers but also results in expression of the transgene in a large population of layer five pyramidal neurons with high specificity even when using a strong non-selective promoter such as CAG. Moreover, because cell transduction takes place at a significant distance from the injection site, this method can help preserve brain tissue for subsequent optical or electrophysiological recordings of neuronal activity.
Introduction
The mammalian brain consists of many inhibitory, excitatory, and modulatory cells interconnected into circuits by trillions of synapses1. One of the central challenges of neuroscience is to decode the role of distinct cell types in the organization and function of brain circuits and behavior. Manipulating genetically defined cells within the brain requires methods to introduce and express transgenes. Viral-based gene delivery systems are by far the most effective and simple method for gene delivery into the central nervous system2. Viral delivery systems are based on replicating viruses (adenoviruses, adeno-associated viruses (AAVs), lentiviruses, and retroviruses) that have the ability to deliver genetic information into a host cell2,3.
AAV-based vectors have now become one of the most widely used tools for the delivery of desired transgenes to cells within the brain, both for purposes of basic neuroscience research and to develop gene therapy for neurological diseases. When compared against other viruses, replication-defective AAVs possess many features that make them ideal vectors for these purposes. Most notably, AAV vectors efficiently transduce nondividing (terminally differentiated) cells such as neurons and glial cells, resulting in high levels of transgene expression in vivo2. The vectors can be easily produced at a high functional titer suitable for in vivo use3,4,5. Importantly, adeno-associated virus-mediated gene delivery in vivo does not produce histopathological alterations and vector-related toxicity6. Unlike adenoviral vectors, in vivo administration of AAV vectors in animal models usually does not elicit host immune responses against transduced cells, enabling stable transgene expression within the brain parenchyma for extended periods of time2,7,8.
Another reason for the popularity of AAV vectors is the broad array of AAV serotypes with unique tissue and cell-type tropisms9,10,11,12,13,14. Distinct capsid proteins expressed by different AAV serotypes result in the use of different cell surface receptors for cell entry and, thus, specific tropisms10,14.
AAV tropism is determined not only by capsid proteins but by many other factors14. It has been shown that AAV serotypes 1, 2, 6, 7, 8, and 9 transduced both neurons and astrocytes in primary culture15,16, but exhibited strong neuronal tropism following intraparenchymal brain injection17,18. The method used for AAV vector preparation can also influence nervous cell tropism, even for the same serotype. For example, CsCl-purified AAV8 possessed strong astroglial tropism following intraparenchymal brain injection, while iodixanol-purified AAV8, injected under identical conditions, transduced only neurons19. AAV tropism may also be affected by the injected dose and volume14. For example, high titer rAAV2/1 efficiently transduced both cortical excitatory and inhibitory neurons, but the use of lower titers exposed a strong preference for transduction of cortical inhibitory neurons20.
Thus, it is not possible to achieve robust cell-type specificity based solely on the capsid serotype. Cell-type specific promoters can be used to overcome the broad natural tropism of the AAV capsid. For example, human synapsin I is used for targeting neurons21, the CaMKII promoter can drive transgene expression in glutamatergic excitatory neurons with high specificity20, the ppHcrt promoter targets hypocretin (HCRT)-expressing neurons in the lateral hypothalamus22, the PRSx8 promoter targets noradrenergic and adrenergic neurons that express dopamine beta-hydroxylase23, and the GFAP promoter can drive astrocyte-specific expression24. However, some cell-specific promoters have weak transcriptional activity and cannot drive sufficient levels of transgene expression25. Furthermore, the short promoters that fit in AAV viral vectors often do not retain cell-type specificity1,26. For example, it has been shown that a CaMKII construct also transduced inhibitory neurons12.
Besides cell-type specificity (tropism), another significant feature of AAVs is transduction efficiency. The various AAV serotypes have different diffusional properties. AAV2 and four viral vectors diffuse less readily through the brain parenchyma and, therefore, mediate transduction over a smaller area17,27. The most widespread neuronal transduction is observed with AAV serotypes 1, 9, and rh.1011,17,18,19,28.
The most popular method to drive the expression of a desired transgene in a particular brain area is to inject the AAV vector directly into the brain region of interest (parenchyma)3. Following intra-parenchymal injection, even AAV serotypes with more effective diffusion through the brain transduce typically only a local area around the injection site 12. Moreover, intraparenchymal injection is an invasive procedure and leads to tissue damage adjacent to the region of interest. Thus, this method of virus injection is unsuitable for some experimental tasks. For example, extensive labeling of cells is highly desirable in experiments aimed at studying cortical neuron functions in freely moving animals, including with the use of one- or two-photon microscopy29,30,31,32.
Here, we describe a new adeno-associated virus injection technique that uses subarachnoid virus infusion to provide widespread transduction of neocortical neurons in adult mice and preserve brain tissue for subsequent optical or electrophysiological recordings of neuronal activity. This method not only ensured widespread transduction of neurons in superficial neocortical layers but resulted in expression of the transgene in a large population of layer five pyramidal neurons with high specificity even when using a strong non-selective promoter such as CAG.
Protocol
Experiments were performed on adult C57Black/6 mice, 2-4 months of age, of both sexes (Pushchino Breeding Center, Branch of the Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry of RAS). Mice were housed in a temperature-controlled vivarium (22 ˚C ± 2 ˚C, 12 h light/dark cycle, lights on at 08.00 h) with food and water ad libitum. All experimental procedures were conducted in accordance with the ARRIVE guidelines and Directive 2010/63/EU for animal experiments. The study protocol was approved by the Ethics Committee of the IHNA RAS (protocol N1 from 01.02.2022). Every effort was made to minimize animal suffering and to ensure the reliability of the results.
1. Preparation for surgery
- Sterilize all surgical instruments prior to the start of surgery. Clean the surgical area using 70% ethanol.
- Check the isoflurane level in the anesthesia system and fill it if needed. Place a clean paper towel on the bottom of the induction chamber.
- Place a heating pad on the stereotaxic frame. Cover the pad with a clean paper towel.
- Prepare a bottle with 2% bleach to collect micropipette tips, tubes, cotton swabs, and other items that come into contact with the virus.
- Remove an aliquot of AAV from the -80 °C freezer and place it on ice to thaw.
2. Syringe preparation
- Clean a 5 µL Hamilton microsyringe with a 33G blunt RN needle. Aspirate and then dispense 70% ethanol. Repeat 3x with fresh ethanol. Rinse the syringe with distilled water to remove the excess ethanol. Repeat 3x.
- Take the plunger out and fill the barrel with Vaseline oil through the flange using an insulin syringe. Make sure that there are no air bubbles in the microsyringe.
NOTE: Trapped air is compressible and affects syringe accuracy and precision. - Put the plunger back in the microsyringe and dispense a drop of oil. Place the syringe into the stereotaxic injector so that the scale is visible for monitoring the volume of solution dispensed.
3. Preparation of mice for surgery
- Weigh a mouse. Place the mouse into the anesthesia induction chamber connected to the isoflurane. Turn on the isoflurane vaporizer set to 5% and adjust the flow rate to 250 mL/min. An adequate depth of anesthesia is achieved within 5 to 7 min.
- To verify the surgical level of anesthesia, check for the absence of whisking and withdrawal reflex during a hind paw painful pinch, and the absence of blinking upon eye contact.
- Switch the vaporizer outflow valve from the induction chamber to the stereotaxic mask. Reduce the isoflurane to 1.8%-2.0% and set the flow rate according to the mouse weight (70-90 mL/min for mice weighing between 25 to 35 g).
- Remove the mouse from the induction chamber and place it in the stereotactic apparatus on top of a heating pad (37 °C) to prevent hypothermia during surgical anesthesia. Place the front teeth in the tooth bar and then mount the animal mask.
- Carefully position the animal on the ear bars. The correct head position in the stereotactic apparatus allows vertical but not lateral movement of the head. Ensure the animal's positioning does not cause distress. Monitor the depth of anesthesia, animal breathing, and body temperature carefully throughout the operation.
- Shave the head from the eyes to behind the ears. Clean the shaved surface of the head by swabbing with 70% ethanol, followed by swabbing with a 5% alcohol solution of iodine.
- Apply ophthalmic gel to prevent eye drying. Apply 4% lidocaine solution topically and dexamethasone (0.02 mL at 4 mg/mL) subcutaneously to prevent surgery-related pain and reduce the possible inflammatory response.
- With a sterile scalpel blade and scissors, make a 4-5 mm long incision along the midline of the head to open the scalp. Start with a small incision between the ears and then expand it using scissors to avoid damage to the skull.
- Swab the surface of the skull with a small amount of 3% hydrogen peroxide to visualize the stereotaxic landmarks: bregma and lambda. Stop the reaction with 0.9% NaCl saline immediately. Scrape away tissues on top of the skull with a bone scraper.
- Mount the pre-prepared motorized injector with the Hamilton syringe on the stereotaxic arm. Direct a surgical light source onto the exposed skull and focus the microscope onto the bregma.
- Looking through the microscope, manipulate the arm of the stereotactic apparatus to center the top of the needle directly over the bregma.
- Using the tip of the needle, align the bregma and lambda horizontally and then move the arm back to bregma and record the coordinates. Use these bregma coordinates and the atlas coordinates of the region of interest to calculate the relative coordinates of the targeted area.
- Move the needle to the targeted area. Lower the needle at the new coordinates and mark this position. Select the site for viral microinjection in close proximity to the target area while considering the spread of the virus.
NOTE: This will avoid damaging the tissue in the region of interest. We used the following coordinates for the region of interest: AP-3.4, ML -2.0, and for viral microinjection: AP-2.0, ML -1.4. The above coordinates are optimal if primary visual cortex neurons are to be infected. - If possible, avoid regions with large blood vessels. Under a surgical microscope, bright, cold light illumination, and 0.9% NaCl saline immersion, large blood vessels in the skull and on the brain surface should become apparent.
4. Virus injection
- Take a sterile dental bur (0.5 - 0.8 mm in diameter). Viewing the surface of the skull through the surgical microscope, drill a small craniotomy manually (by hand) or using a microdrill. Take care not to put excessive pressure on the skull.
- Move the arm out of the way to prevent damage to the microsyringe needle during the drilling of the craniotomy (hole). Apply 0.9% NaCl saline immersion and pause intermittently to avoid heating the bone and damaging the dura mater. Use pressurized air to blow away bone dust.
NOTE: The following types of carbide dental burs are suitable: pear-shaped (preferably), rounded cylinder, and round.- When thinning the bone with the bur, ensure the thinning is uniform across the entire circumference. This will make it easier to remove the bone without damaging the dura mater. To do this, use a bur with a round tip initially and then a bur with a flat tip. Keep the bur perpendicular to the bone; otherwise, one side will be thinner than the other.
NOTE: Additionally, the smaller the size of the bur and the smaller the craniotomy performed, the more complex the manipulation will be. It is recommended that a craniotomy of smaller diameter (0.5-0.6 mm) be performed when further using the animal for in vivo work. If planning to use the animal's brain for ex vivo work, a larger craniotomy diameter is acceptable to simplify the procedure.
- When thinning the bone with the bur, ensure the thinning is uniform across the entire circumference. This will make it easier to remove the bone without damaging the dura mater. To do this, use a bur with a round tip initially and then a bur with a flat tip. Keep the bur perpendicular to the bone; otherwise, one side will be thinner than the other.
- When the thinned bone is soft and transparent, stop drilling. When a sufficiently large indentation forms in the bone, frequently stop the rotation and monitor the thickness of the bone. The appearance of cracks in the bone indicates that thinning is sufficient.
- Bathe the hole with sterile saline and then remove the excess saline with a cotton swab. Remove the remaining layer of bone using a 27G needle with a hook-shaped tip and/or using fine-tipped tweezers. Avoid damaging the dura.
- Cover the surface of the skull with a sterile piece of paper towel moistened with saline. Place a piece of clean, transparent film on the mouse skull surface above the paper.
- Move the stereotaxic arm back and position the microsyringe needle in place directly above the film. Dispense the excess oil until a volume of 2 µL is reached.
NOTE: The final volume of the oil may vary according to the volume of the micro syringe. We used a 5 µL 75-RN Hamilton micro syringe. - Pipette a volume of virus equal to the injected volume + 2 µL onto a piece of transparent film.
- Looking through the surgical microscope, lower the arm down until the tip of the needle is in the center of the drop of the virus. Load the virus into the microsyringe using a motorized injector. Dispose of the tip of the micropipette and transparent film into the bottle with 2% bleach.
- Remove the paper moistened with saline from the surface of the skull and dry the skull with a cotton swab. Use the stereotaxic arm to position the needle above the insertion site.
- Dispense a drop of the virus to make sure that the needle is not clogged. Make a small slit in the dura using a 30 G needle with a hook-shaped tip.
NOTE: It is important to make the smallest possible slit in the dura mater, allowing the needle to enter without leaving a gap between the needle and the dura mater, from which the virus could leak. - Lower the needle of the syringe down to the dura and make the appropriate calculations for depth. Estimate the cortical depth of the insertion relative to the point at which the needle first touched the surface of the cortex.
- Slowly insert the needle tip into the cortex to a depth of 300 µm. Wait 2-3 min to allow the dura to adhere to the needle, and then slowly retract the needle to a depth of 200 µm. Wait another 2-3 min to allow the brain tissue to settle. This results in the needle pulling the dura up, creating a subdural space for the virus to spread.
NOTE: To minimize cortical damage, use a 33 G needle or lower G needles. Keep in mind that the smaller the tip's inner diameter is, the higher the chance of tissue backflow clogging it. It is important to use a blunt needle instead of beveled because a blunt needle expels a more controlled drop of viral solution.- If the needle does not pass through the dura mater when lowered to 150-200 µm and instead bends it, stop lowering. Lift the needle and make a slightly larger incision in the dura mater, then try lowering the needle again.
- If cerebrospinal fluid starts leaking actively from the incision while lowering the needle, wait until it stops leaking; otherwise, it is hard to control the needle passing through the dura. Blot the excess cerebrospinal fluid with a tissue tip. Once the fluid stops flowing, lift the needle and try again.
- Begin injecting the viral suspension at a rate of 0.06 µL/min while monitoring the volume dispensed. Inject 1 µL of virus. Inject the virus at a single depth in order not to break the seal between the needle and cortex tissue.
- Troubleshooting: At the beginning of the injection, virus backflow to the brain surface may occur. Stop injecting, wait 2-3 min, and then continue the virus injection. Repeat these actions (steps) until the virus backflow stops. Virus and cerebrospinal fluids will stick to the needle and prevent backflow.
- Another way to stop virus backflow, put a small amount of agarose on the surface of the cortex. It will suppress the pulsation of the cortex and will also help to seal the needle. But make sure that the pipette is not clogged by the agarose.
- Since the needle may not completely pass through the dura mater when lowered, but only partially, one side of the needle may not fit tightly against the dura, which can cause backflow of the virus. In this case, slowly withdraw the needle from the tissue and try lowering it again.
- Once the infusion is complete, keep the needle at the target location for an additional 10 min. This allows the virus to disperse away from the injection site. Retract the needle out of the brain slowly to prevent the virus from flooding back out of the needle tract.
- Once the needle withdrawal is completed, check that it is not clogged by dispensing a small drop of virus.Close the skin incision using 5-0 absorbable or non-absorbable sutures.
5. Post-operative care
- After closing the incision, apply antibacterial ointment topically. Inject a mix of saline (5 mL/kg) and 5% glucose (5 mL/kg) subcutaneously to prevent dehydration and facilitate recovery after anesthesia. Inject ketoprofen intramuscularly (2.5 mg/kg) to reduce pain.
- Place the animal in a fresh cage on top of a heating pad and monitor until it recovers from anesthesia. After the mouse is reactive, place the animal in their home cage.
6. Histology
- No earlier than 21 days after virus injection deeply anesthetize mice with isoflurane as described in step 3.
- Make a midline incision (10 -15 mm) along the thoracic region using narrow scissors and expose the chest cavity. Carefully separate the diaphragm and open the chest using scissors.
- Insert a 22G 1 1/2 needle into the left ventricle and make an incision in the right atrium with scissors. Perfuse with 50 mL of pre-chilled 100 mM Phosphate Buffered Saline (PBS), followed by 100 mL of pre-chilled 10% buffered formalin.
- Carefully extract the entire mouse brain. To do this, make a sagittal incision along the center of the scalp using scissors. Next, remove the cranial bone piece by piece using bone pliers with flat tips starting at the intersection of the sagittal and lambdoid sutures and working forward to the nasal bone. When the brain is exposed, cut through the olfactory bulbs, ease the brain out with a curved spatula, and drop it into a 50 mL tube containing 10% buffered formalin.
- Fix the brain overnight. Mount the brain tissue on a metal platform of a Vibratome using tissue adhesive. Cut frontal sections at a thickness of 50 µm using carbon steel blades.
7. Immunostaining
- Day 1
- Wash the brain slices 3x for 5 min each with 1x PBS containing 0.3% Triton X-100 (PBS-T). Incubate the sections in PBS-T for 20 min at room temperature (RT).
- Block nonspecific binding for 1 h at RT using a blocking buffer composed of 5% Normal Goat Serum (NGS) and 0.3% Triton X-100 in 1x PBS.
- Incubate the sections overnight at 4 °C with primary antibodies against parvalbumin or calbindin diluted 1:500 in the blocking buffer (5% NGS and 0.3% Triton X-100 in 1x PBS).
- Day 2
- Wash the sections 3x for 10 min in PBS-T. Incubate in the dark for 2 h at RT with secondary antibodies (Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 546) diluted 1:500 in the blocking buffer (5% NGS and 0.3% Triton X-100 in 1x PBS).
- Wash the sections 3x for 5 min each in 1x PBS. Transfer brain slices onto glass slides using a soft brush.
- Immediately add 0.1 mL of antifade mounting medium to each section. Cover the slices with a 22 mm x 50 mm coverslip.
- Configure the confocal microscope to a magnification of 20x or 60x (A/1.4, oil). Use 488 nm and 594 nm wavelength lasers to acquire multi-channel images of the brain regions of interest.
Results
In a pilot series of experiments, we used the traditional intracortical injection method to transduce layer five pyramidal neurons in the mouse neocortex by AAV2 carrying the fast channelrhodopsin (oChIEF) gene fused with EGFP fluorescent protein under the CaMKII promoter. Consistent with the characteristic feature of AAV212, we obtained a relatively small area of infection, not exceeding 1 mm in width (Figure 1A). However, in some experiments, we observed unusually large spreading of AAV2, in some cases covering the neocortex of more than half of the brain hemisphere (Figure 1B). We hypothesized that such widespread virus distribution may occur when the virus enters the subarachnoid space and the cerebrospinal fluid (CSF) stream spreads the viral vector across the brain surface. We noted that this happens when the injection needle insertion depth is small (<200 µm) and the size of the hole in the dura mater exactly matches the diameter of the needle, preventing the suspension of viral particles from backflow. In order to visualize this process, we added red fluorescent nanoparticles to the injected suspension of viral particles (n = 3 mice). At 3 weeks after injection, mice were perfused transcardially with 10% buffered formalin, and the brains were carefully removed from the skull without damaging the dura mater. Examination of whole brains under an epifluorescence binocular microscope revealed the widespread distribution of red fluorescent particles that slightly exceeded the area of neuronal infection visible in the green fluorescence channel (Figure 2). Analysis of sagittal sections from the brains of these mice showed that fluorescent particles were located in a thin layer along the pia mater, without penetrating deep into the brain parenchyma, while Venus-expressing neurons, as in previous experiments, were found in large numbers in layers 2/3 and 5.
In addition to the AAV2_CaMKII_oChIEF EGFP virus (used at a concentration of 1.49 x 1012 vg/mL), we also performed subarachnoid administration of the AAV2_CaMKII_Venus (7.31 x 1012 vg/mL) and AAV2_CAG_GCamp6s (7.3 x 1013 vg/ml) viruses and obtained similar results. This is important because, as has been shown, viruses of the same serotype can provide different sizes of transduction area depending on the promoter and target gene used12.
To systematically compare transduction areas after traditional and subarachnoid administration of viruses, we calculated the size of the infection area in the mediolateral and rostrocaudal directions in serial 50 µm thick sections of brains. It was found that subarachnoid administration of the virus led to an almost fourfold increase in the infection area, compared with intraparenchymal administration (1.7 ± 0.52 mm (n = 15 mice) versus 0.46 ± 0.22 mm (n = 6 mice), p < .00001, t-test in the mediolateral direction and 2.35 ± 0.8 mm (n=14 mice) versus 0.84 ± 0.29 mm (n = 6 mice) p < .0003, t-test in the rostrocaudal direction; Figure 3A).
Microscopic observation of brain slices from mice transduced by subarachnoid virus injection revealed very widespread transduction in layers 2/3 and 5, while there were virtually no transduced cells in layers 4 and 6 (Figure 3B). In layer 4, only fluorescent dendrites of layer 5 pyramids were clearly visible (Figure 3B). Fluorescent axons were traced in layer 6 and white matter (Figure 3C). Such a transduction pattern after subarachnoid injection could be due to virus diffusion from the subarachnoid space into layer 1 of the cortex (maybe deeper), where neuronal dendrites capture it. Thus, only neurons that have vigorous branching in the upper layers are infected. It is known that GABAergic interneurons predominantly branch locally. If our hypothesis is correct, then subarachnoid administration of the virus should lead to the transduction of interneurons in the supragranular layers but not in the subgranular layers. To test the hypothesis, we performed immunochemical staining of brain sections from mice after subarachnoid injection of the AAV2_CaMKII_Venus virus with antibodies to markers of two different functional classes of GABAergic interneurons: parvalbumin and calbindin.
To determine the number of interneurons transduced by subarachnoid injection of the virus, we performed a morphometric analysis counting the total number of transduced cells (green staining), the number of immunopositive neurons (red staining) and the number of double-labeled cells on a 750 x 750 µm section (50 µm section thickness).
On brain slices stained with antibodies against parvalbumin, in the supragranular layers, the number of neurons with green labeling averaged 57.4 ± 9.8, parvalbumin-positive interneurons with red labeling 9.6 ± 3.8, of which 4.1 ± 2.4 (42.7%) were double-labeled (n = 10 preparations). In contrast, we counted 14 ± 4.8 virus-transduced neurons and 19.1 ± 4.5 parvalbumin-positive neurons in layer 5 of the neocortex and did not detect any double-labeled cells (n = 10 preparations).
When we examined sections immunochemically stained for calbindin, we found that in the supragranular layers, there was an average of 21.1 ± 4.5 virally transduced neurons and 6.1 ± 2.6 calbindin-positive cells, of which 4.2 ± 1.9 cells carried both labels (69.1%; n = 10 preparations). In layer 5, we counted 19 ± 2.1 transduced neurons, 15.9 ± 5.7 calbindin interneurons, of which 1.1 ± 1.5 (6.9%) showed double staining. However, it should be noted that 100% of the double-labeled neurons in layer 5 had a clearly visible pyramidal shape, which may indicate the presence of calbindin-positive pyramidal neurons or some non-specificity of the antibodies. Thus, no true calbindin interneurons transduced by subarachnoid injection of the virus were observed in layer 5.
Therefore, while parvalbumin and calbindin-positive cells were indeed present among the transduced neurons in layer 2/3 (Figure 4A,C), no transduced interneurons were detected in layer 5, and all EGFP-expressing cells were visually identified as pyramidal neurons (Figure 4B,D).

Figure 1: Comparison of AAV2 transduction area after conventional intracortical and subarachnoid viral injections. (A) The spread of CaMKII_oChieff_EGFP constructs 21 days after injection into the brain parenchyma to a depth of 500 - 600 µm. (B) The spread of CaMKII_oChieff_EGFP in the other hemisphere of the same animal following subarachnoid administration. Frontal sections at a distance of 200-300 µm in the rostrocaudal direction from the injection site are shown. Scale bar - 1 mm. Please click here to view a larger version of this figure.

Figure 2: Photograph of a whole brain with intact dura matter showing the spread of AAV2_CaMKII_Venus viruses and red FluoSpheres injected subarachnoidally. (A) Fluorescence of red nanoparticles that were added to the injected virus suspension showing the physical spread of the injected volume in the subarachnoid space. (B) The same brain hemisphere in the green fluorescence channel shows the Venus expression area. The injection site is indicated by arrows. In addition, the location of the lambda is marked. Scale bar - 1 mm. Please click here to view a larger version of this figure.

Figure 3: Subarachnoid injection of the virus results in widespread infection of neurons of layers 2/3 and 5 of the neocortex. (A) Comparison of the transduction areas (in the mediolateral (m/l) and rostrocaudal (r/c) directions) after intracortical (i) and subarachnoid (s) virus injection. The bars represent the mean; the whiskers denote the standard deviation (**** - p < 0.0001; *** - p < 0.001; t-test). (B) Confocal micrograph showing Venus expression in the mouse neocortex after subarachnoid injection of AAV2_CaMKII_Venus viruses. Layer boundaries are shown schematically (L1 - L6). (C) A fragment of image (B), shown with different brightness and contrast settings to demonstrate fluorescent axons traveling across layer 6 and the white matter (WM). The scale bar is 100 µm. Please click here to view a larger version of this figure.
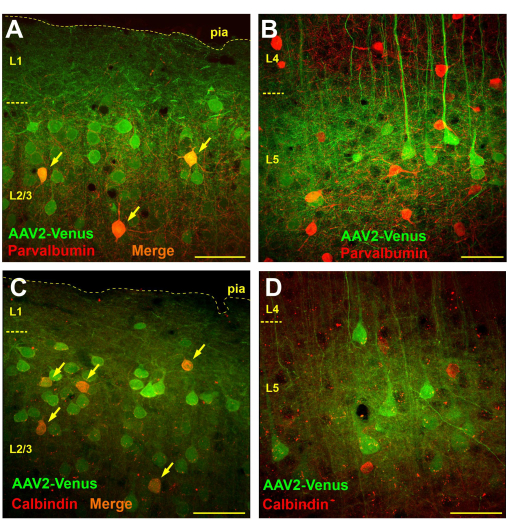
Figure 4: Subarachnoid virus injection results in transduction of interneurons in supra- but not in subgranular layers of the neocortex. (A, B) Immunochemical staining of brain sections from a mouse transduced by subarachnoid injection of the AAV2_CaMKII_Venus virus with antibodies to parvalbumin (Alexa594-conjugated secondary antibodies). Neurons carrying both green and red labels appear orange (indicated by arrows). (C, D) Micrographs of a brain section of a mouse after subarachnoid injection of AAV2_CaMKII_Venus stained with antibodies to calbindin. Note the absence of double-labeled neurons in L5. The scale bar is 50 µm. Please click here to view a larger version of this figure.
Discussion
We have developed a new method for transducing mouse neocortical neurons by injecting a suspension of AAV2 viral particles into the subarachnoid space of the brain. This provides widespread virus distribution, almost four-fold greater than the tissue volume infected when the same amount of virus is injected directly into the brain parenchyma.
Injection of virus vectors directly into the cerebrospinal fluid (CSF) via different routes (e.g., intracerebroventricular, intrathecal, or intracisternal) is a popular strategy for widespread gene delivery throughout the CNS33,34,35. However, intracerebroventricular or intrathecal administration of AAV2 vectors in the adult brain results in limited brain transduction due to their high affinity for the ependymal cells28,33,36,37. Ependymal cells are found as a monolayer that lines the third and fourth ventricles and the central canal of the spinal cord38. The presence of tight junctions between ependymal cells in the ventricle is a significant barrier for some AAV serotypes, which must pass through these cells from the ventricle to have intraparenchymal spread39.
In our work, the injection of the AAV2 viral vector into the subarachnoid space through the brain surface resulted in widespread transduction of neocortical neurons in adult mice. There is evidence that the pia matter has different structures in different areas of the CNS. The membrane in the spinal pia mater (which surrounds the spinal cord) is much thicker than the cranial pia mater (which surrounds the brain) due to the two-layered nature of the pia membrane40. It has also been shown that ventricular CSF enters the brain parenchyma minimally, whereas subarachnoid CSF rapidly enters the brain parenchyma along paravascular spaces41. It is, therefore, likely that different routes of delivery of the viral vector into the CSF may give different results.
It should be noted that intra-CSF injections of some AAV variants are associated with certain side effects. Intrathecal or intracerebroventricular delivery of AAV9 has been shown to result in gene expression not only in the CNS but also in peripheral organs42,43. For more efficient transduction of neurons throughout the brain, high vector doses are required. For example, adult Sprague-Dawley rats received a unilateral injection of AAV9 into the lateral ventricle in three doses: 3.1 µL, 15.5 µL, and 77.5 µL42. Mice received AAV9 in total volume of 10 µL in the cisterna magna43. The benefit of our method of virus injection is that we used a lower vector volume (1 µL) than those used for the intrathecal or intracerebroventricular delivery of AAV. A lower volume of the virus significantly decreases the risk of viral expression outside the brain and toxicity as well.
Although we did not examine viral expressions outside the brain, it is highly likely that subarachnoid administration of AAV2 through the brain surface resulted in viral expression exclusively in the brain (specifically in the neocortex). Our assumption is based on the following reasons. AAV9 is a recombinant adeno-associated virus that can cross the blood-brain barrier (BBB) and is commonly used for global CNS transduction37,44. However, BBB penetration and transduction of brain tissue are limited with AAV245. Furthermore, analysis of mouse brain slices after subarachnoid injection of the virus showed that transduced cells were exclusively located in the neocortex of the ipsilateral (injected) hemisphere. No transduced cells were found in the contralateral neocortex or other brain structures, suggesting that transduction of peripheral organs by this method of injection is very unlikely.
In addition to a large infection area, the method of subarachnoid virus injection allows selective transduction of layer five pyramidal neurons, even with the use of strong non-selective promoters such as CAG. It is well known that ensuring selective expression even with specific promoters is quite difficult1,26. For example, the CaMKII promoter we used should theoretically preferentially infect glutamatergic neurons. However, as shown in the results here and other studies, when it is used, transduction of other types of cells also occurs, in particular, GABAergic interneurons46. Moreover, because with subarachnoid injections, cell transduction takes place at a significant distance from the injection site, this method helps preserve brain tissue for subsequent optical or electrophysiological recordings of neuronal activity. We have successfully used subarachnoid virus injections in experiments with optogenetic stimulation and extracellular recording of activity of L5 pyramidal neurons of the mouse visual cortex in vivo32.
This work is a spin-off of our large-scale study on the mechanisms of plasticity in the visual cortex, in which we used AAV2 to express channel rhodopsin in the pyramidal neurons of the mouse visual cortex32. With this serotype, we gained a lot of statistics and, in fact, developed the method of subarachnoid injection. In pilot experiments, we also tried subarachnoid injection of the virus with serotype 2/9 and obtained similar results, although we did not perform detailed morphometric analysis in this case. Unfortunately, it is impossible to predict how other serotypes will behave after subarachnoid administration and which virus serotype will provide the largest transduction area; this can only be determined empirically, which requires a considerable amount of work. In this work, we have convincingly shown that AAV2 can be injected into the subarachnoid space of the adult mouse brain, resulting in widespread transduction with selective expression of the target gene in layer five pyramidal neurons of the neocortex and non-selective expression in supragranular layers.
The most critical step in using this method of subarachnoid virus injection is to ensure the optimal size of the hole in the dura matter, which must exactly match the diameter of the injection needle. The dura should tightly encircle the needle and, therefore, prevent the backflow of the virus during the injection. We have called this method the subarachnoid injection, but it is not clear if, in addition to the subarachnoid space, the virus also enters and spreads into the subdural space (the space between the arachnoid and the dura mater). It is also not clear whether this method would work in other animals, particularly in rats, or with other AAV serotypes.
Previously, Xinjian Li and colleagues described a neuronal transduction method based on viral infusion at the cortical surface. They used a wide-diameter glass pipette at the cortical surface for infusing the viral calcium reporter AAV-GCaMP6 into the cortex. Using this method, viral particles, presumably, similarly to this case, enter the upper layers of the neocortex, where they are captured by neurons. The authors found that cortical surface virus infusion efficiently labeled neurons in the superficial layers while avoiding deep layer neurons47. It is not entirely clear why the cited work did not show the transduction of layer five pyramidal neurons, similar to what we observed in our study.
Our hypothesis that following subarachnoid administration, the virus is captured by the dendrites of neurons branching in the supragranular layers has one noticeable weakness. In addition to the large pyramids of layer five, layer four pyramidal neurons48 and the claustrum-projecting L6 pyramidal cells49 in the mouse visual cortex have dendrites that reach layer one. Therefore, it is not clear why these cells are not transduced with subarachnoid virus injection. One possible explanation is that only those cells whose dendrites are thick enough to transport the viral particle to the cell body are infected - i.e., L5 pyramids. It has been shown that in the visual cortex of mice, layer four pyramidal neurons send only one thin dendrite to the layer one of the neocortex48. However, further studies are needed to determine the cause of the observed infection pattern after subarachnoid virus injection.
Disclosures
The authors declare no conflicts of interest.
Acknowledgements
The work was carried out with financial support from the Russian Science Foundation, grant 20-15-00398P.
Materials
| Name | Company | Catalog Number | Comments |
| Equipment | |||
| 10 µL Gastight Syringe Model 1701 RN (5 uL 75 RN Hamilton microsyringe) | Hamilton Company | Part/REF # 7634-01, Hamilton or cat no. HAM7634-01, Merck | |
| 33 G RN needle, point style 3 | Hamilton Company | Part/REF # 7803-05, Hamilton | |
| Binocular Microscope | Nikon or Micromed | Model MC-4 ZOOM | |
| Cerna-based laser scanning confocal microscope | ThorLabs | ||
| Cold light source | RWD | Model 76312 | |
| Leica VT1000 S Vibrating blade microtome | Leica Biosystems | 76001-014 | |
| Low-Flow Anesthesia System with starter kit | Kent Scientific Corporation | 13-005-111 (Model SomnoSuite) | |
| Mechanical Pipette 0.1 – 2.5 µL Eppendorf Research plus | Eppendorf | 3123000012 | |
| Mechanical Pipette 10 – 100 µL Eppendorf Research plus | Eppendorf | 3123000047 | |
| Mice Shaver | RWD | Model CP-5200 | |
| Microdrill with drill bits (0.5 mm, round) | RWD | 78001, 78040 | |
| or Desctop Digital Stereotaxic Instrument, Mouse anesthesia Mask, Mouse ear bars (60 Deg) | RWD | Models 68027, 68665, 68306 | |
| Pressurized air | KUDO | ||
| Single Channel Manual Pipette 0.5-10 µL | RAINN | 17008649 | |
| Small Animal Stereotaxic Instrument | KOPF | Model 962 | |
| Stereotaxic Injector | Stoelting | 10-000-004 | |
| Surgical Instruments (Tools) | |||
| 30 G dental needle (Ni-pro) | Biodent Co. Ltd. | To slit the dura | |
| Bone scraper | Fine Science Tools | 10075-16 | |
| Dental bur | DRENDEL + ZWEILING | For craniotomy; Shape: pear shaped/round end cylinder/round; Tip Diameter: 0.55-0.8 mm diameter | |
| Needle holder (Halsey Micro Needle Holder) | Fine Science Tools | 12500-12 | |
| Polypropylene Surgical Suture or Surgical Suture Vicryl (5-0, absorbable) | Walter Products (Ethicon) | S139044 (W9442) | |
| Scalpel handle (#3) with scalpel blades (#11) | Fine Science Tools | 10003-12, 10011-00 | |
| Scissors (Extra Narrow Scissors) | Fine Science Tools | 14088-10 | to cut the skin |
| Scissors (Fine Scissors) | Fine Science Tools | 14094-11 | to cut suture |
| Surgical suture PROLENE (Polyproptlene) | Ethicon (Johnson & Johnson) | ||
| Tweezers (Forceps #5) | Fine Science Tools | 11252-20 | |
| Tweezers (Polished Inox Forceps) | Fine Science Tools | 11210-20 | |
| Disposables | |||
| 1 mL insulin syringe | SITEKMED | To load vaseline oil into a microsyringe, to administer drugs | |
| Cell Culture Plate | SPL Life Science | ||
| Cotton swabs | |||
| Cover Glasses | Fisher Scientific | 12-545E | |
| Insulin syringe needle (27 G) | SITEKMED | To remove debries from a hole (craniotomy) | |
| Lint-free wipes CLEANWIPER | NetLink | ||
| Microscope Slides | Fisher Scientific | 12-550-15 | |
| Paper towels | Luscan | ||
| Parafilm | StatLab | STLPM996 | |
| Sterile Surgical Gloves | Dermagrip | ||
| Drugs/Chemicals (Reagents) | |||
| 10% buffered formalin or 4% paraformaldehyde | Thermo Scientific Chemicals | J61899.AK | |
| Alcohol solution of iodine (5%)) | Renewal | ||
| Antibiotic ointment Baneocin (bacitracin + neomycin) | Sandoz | Antibacterial agent for external use | |
| Aqua Polymount | Poly-sciences | 18606-20 | |
| Carbomer Eye Gel Vidisic (Ophthalmic gel) | BAUSCH+LOMB (Santen) | ||
| Carboxylate-Modified FluoSphere Microspheres (red) | Thermo Fisher Scientific | F-8801 | |
| Dexamethasone (4 mg/mL) | Ellara (KRKA) | Synthetic glucocorticoid | |
| Distilled H2O | |||
| Ethanol (70%) | |||
| Flexoprofen 2.5% (Ketoprofen) | VIC | Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) | |
| Glucose solution 5% | Solopharm | ||
| Goat anti-Rabbit IgG (H+L) Cross-Adsorbed, Alexa Fluor 546 | Thermo Fisher Scientific | A-11010 | |
| Isoflurane | Karizoo | ||
| lidocaine solution (2 % / 4%) | Solopharm | ||
| Normal Goat Serum (NGS) | Abcam | ab7481 | |
| Phosphate Buffered Saline (PBS) | Eco-servis | ||
| Rabbit Anti-Parvalbumin Antibody | Merck Millipore | AB15736 | |
| Rabbit Recombinant Monoclonal anti-Calbindin antibody | Abcam | ab108404 | |
| Saline (0.9% NaCl in H2O) | Solopharm | ||
| Triton X-100 | Sigma-Aldrich | 50-178-1844 | |
| Vaseline oil | Genel |
References
- Luo, L., Callaway, E. M., Svoboda, K. Genetic dissection of neural circuits. Neuron. 57 (5), 634-660 (2008).
- Sena-Esteves, M., Gao, G. Introducing Genes into Mammalian Cells: Viral Vectors. Cold Spring Harb Protoc. 2020 (8), (2020).
- Kootstra, N. A., Verma, I. M. Gene Therapy with Viral Vectors. Ann Rev Pharmacol Toxicol. 43 (1), 413-439 (2003).
- Paterna, J. C., Feldon, J., Büeler, H. Transduction profiles of recombinant adeno-associated virus vectors derived from serotypes 2 and 5 in the nigrostriatal system of rats. J Virol. 78 (13), 6808-6817 (2004).
- Monahan, P. E., Samulski, R. J. Adeno-associated virus vectors for gene therapy: more pros than cons. Mol Med Today. 6 (11), 433-440 (2000).
- Daya, S., Berns, K. I. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 21 (4), 583-593 (2008).
- Jooss, K., Chirmule, N. Immunity to adenovirus and adeno-associated viral vectors: implications for gene therapy. Gene Ther. 10 (11), 955-963 (2003).
- Naso, M. F., Tomkowicz, B., Perry, W. L., Strohl, W. R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs. 31 (4), 317-334 (2017).
- Gao, G., Vandenberghe, L., Wilson, J. New Recombinant Serotypes of AAV Vectors. Curr Gene Ther. 5 (3), 285-297 (2005).
- Büning, H., Perabo, L., Coutelle, O., Quadt-Humme, S., Hallek, M. Recent developments in adeno-associated virus vector technology. J Gene Med. 10 (7), 717-733 (2008).
- Aschauer, D. F., Kreuz, S., Rumpel, S. Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PloS One. 8 (9), e76310-e76310 (2013).
- Watakabe, A., et al. Comparative analyses of adeno-associated viral vector serotypes 1, 2, 5, 8 and 9 in marmoset, mouse and macaque cerebral cortex. Neurosci Res. 93, 144-157 (2015).
- de Solis, C. A., et al. Adeno-associated viral serotypes differentially transduce inhibitory neurons within the rat amygdala. Brain Res. 1672, 148-162 (2017).
- Castle, M. J., Turunen, H. T., Vandenberghe, L. H., Wolfe, J. H. Controlling AAV Tropism in the Nervous System with Natural and Engineered Capsids. Meth Mol Biol. 1382, 133-149 (2016).
- Howard, D. B., Powers, K., Wang, Y., Harvey, B. K. Tropism and toxicity of adeno-associated viral vector serotypes 1, 2, 5, 6, 7, 8, and 9 in rat neurons and glia in vitro. Virology. 372 (1), 24-34 (2008).
- Royo, N. C., et al. Specific AAV serotypes stably transduce primary hippocampal and cortical cultures with high efficiency and low toxicity. Brain Res. 1190, 15-22 (2008).
- Burger, C., et al. Recombinant AAV Viral Vectors Pseudotyped with Viral Capsids from Serotypes 1, 2, and 5 Display Differential Efficiency and Cell Tropism after Delivery to Different Regions of the Central Nervous System. Mol Ther. 10 (2), 302-317 (2004).
- Cearley, C. N., Wolfe, J. H. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol Ther. 13 (3), 528-537 (2006).
- Klein, R. L., Dayton, R. D., Tatom, J. B., Henderson, K. M., Henning, P. P. AAV8, 9, Rh10, Rh43 vector gene transfer in the rat brain: effects of serotype, promoter and purification method. Mol Ther. 16 (1), 89-96 (2008).
- Nathanson, J. L., Yanagawa, Y., Obata, K., Callaway, E. M. Preferential labeling of inhibitory and excitatory cortical neurons by endogenous tropism of adeno-associated virus and lentivirus vectors. Neuroscience. 161 (2), 441-450 (2009).
- Zhang, F., et al. Multimodal fast optical interrogation of neural circuitry. Nature. 446 (7136), 633-639 (2007).
- Adamantidis, A. R., Zhang, F., Aravanis, A. M., Deisseroth, K., de Lecea, L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 450 (7168), 420-424 (2007).
- Abbott, S. B. G., et al. Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci. 29 (18), 5806-5819 (2009).
- Lawlor, P. A., Bland, R. J., Mouravlev, A., Young, D., During, M. J. Efficient gene delivery and selective transduction of glial cells in the mammalian brain by AAV serotypes isolated from nonhuman primates. Mol Ther. 17 (10), 1692-1702 (2009).
- Adamantidis, A. R., Zhang, F., de Lecea, L., Deisseroth, K. Optogenetics: Opsins and Optical Interfaces in Neuroscience. Cold Spring Harb Protoc. 2014 (8), (2014).
- Zhang, F., et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc. 5 (3), 439-456 (2010).
- Passini, M. A., Watson, D. J., Wolfe, J. H. Gene Delivery to the Mouse Brain with Adeno-Associated Virus. Methods Mol Biol. 246, 225-236 (2004).
- Davidson, B. L., et al. Recombinant adeno-associated virus type 2, 4, and 5 vectors: Transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci. 97 (7), 3428-3432 (2000).
- Peters, A. J., Chen, S. X., Komiyama, T. Emergence of reproducible spatiotemporal activity during motor learning. Nature. 510 (7504), 263-267 (2014).
- Harris, K. D., Shepherd, G. M. G. The neocortical circuit: themes and variations. Nat Neurosci. 18 (2), 170-181 (2015).
- Carrillo-Reid, L., Yang, W., Kang Miller, J., Peterka, D. S., Yuste, R. Imaging and Optically Manipulating Neuronal Ensembles. Ann Rev Biophy. 46 (1), 271-293 (2017).
- Smirnov, I. V., et al. Plasticity of Response Properties of Mouse Visual Cortex Neurons Induced by Optogenetic Tetanization In Vivo. Curr Issues Mol Biol. 46 (4), 3294-3312 (2024).
- Snyder, B. R., et al. Comparison of Adeno-Associated Viral Vector Serotypes for Spinal Cord and Motor Neuron Gene Delivery. Human Gene Ther. 22 (9), 1129-1135 (2011).
- Schuster, D. J., et al. Biodistribution of adeno-associated virus serotype 9 (AAV9) vector after intrathecal and intravenous delivery in mouse. Front Neuroanat. 8, 42 (2014).
- Bedbrook, C. N., Deverman, B. E., Gradinaru, V. Viral Strategies for Targeting the Central and Peripheral Nervous Systems. Ann Rev Neurosci. 41 (1), 323-348 (2018).
- Lo, W. D., et al. Adeno-Associated Virus-Mediated Gene Transfer to the Brain: Duration and Modulation of Expression. Human Gene Ther. 10 (2), 201-213 (1999).
- Gray, S. J., Nagabhushan Kalburgi, S., McCown, T. J., Ju de Samulski, R. Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther. 20 (4), 450-459 (2013).
- Del Bigio, M. R. Ependymal cells: biology and pathology. Acta Neuropathol. 119 (1), 55-73 (2009).
- Daci, R., Flotte, T. R. Delivery of Adeno-Associated Virus Vectors to the Central Nervous System for Correction of Single Gene Disorders. Int J Mol Sci. 25 (2), 1050 (2024).
- Dasgupta, K., Jeong, J. Developmental biology of the meninges. Genesis. 57 (5), e23288-e23288 (2019).
- Mestre, H., Mori, Y., Nedergaard, M. The Brain's Glymphatic System: Current Controversies. Trends Neurosci. 43 (7), 458-466 (2020).
- Donsante, A., et al. Intracerebroventricular delivery of self-complementary adeno-associated virus serotype 9 to the adult rat brain. Gene Ther. 23 (5), 401-407 (2016).
- Hordeaux, J., et al. Long-term neurologic and cardiac correction by intrathecal gene therapy in Pompe disease. Acta Neuropathol Comm. 5 (1), 66 (2017).
- Deverman, B. E., et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat Biotechnol. 34 (2), 204-209 (2016).
- Zhang, H., et al. Several rAAV vectors efficiently cross the blood-brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system. Mol Ther. 19 (8), 1440-1448 (2011).
- Radhiyanti, P. T., Konno, A., Matsuzaki, Y., Hirai, H. Comparative study of neuron-specific promoters in mouse brain transduced by intravenously administered AAV-PHP.eB. Neurosci Lett. 756, 135956 (2021).
- Li, X., et al. Skin suturing and cortical surface viral infusion improves imaging of neuronal ensemble activity with head-mounted miniature microscopes. J Neurosci Meth. 291, 238-248 (2017).
- Scala, F., et al. Layer 4 of mouse neocortex differs in cell types and circuit organization between sensory areas. Nat Comm. 10 (1), 4174 (2019).
- Thomson, A. M. Neocortical layer 6, a review. Front Neuroanat. 4, 13 (2010).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved