Method Article
Multiscale Investigations of Cortical Processing by Integrating Laminar Polytrodes and Optogenetics with Micro Electrocorticography in Rodents
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
Here, we present two protocols for high-density micro-electrocorticography (µEcoG) recording in rats and mice, including surgical, implantation, and recording methods. µECoG recordings are performed in combination with either laminar polytrode recording in the rat auditory cortex or with optogenetic manipulation of neural activity in the mouse somatosensory cortex.
Streszczenie
Electrocorticography (ECoG) is a methodological bridge between basic neuroscience and understanding human brain function in health and disease. ECoG records neurophysiological signals directly from the cortical surface at millisecond temporal resolution and columnar spatial resolution over large regions of cortical tissue simultaneously, making it uniquely positioned to study both local and distributed cortical computations. Here, we describe the design of custom, high-density micro-ECoG (µECoG) devices and their use in two procedures. These grids have 128 low-impedance electrodes with 200 µm spacing fabricated on a clear polymer substrate with perforations between electrodes; these features enable simultaneous µECoG recording with laminar polytrode recordings and optogenetic manipulations. First, we present a protocol for combined epidural µECoG recording over the whisker somatosensory cortex of mice with optogenetic manipulation of specific genetically defined cortical cell types. This allows causal dissection of the distinct contributions of different neuronal populations to sensory processing while also monitoring their specific signatures in µECoG signals. Second, we present a protocol for acute experiments to record neural activity from the rat auditory cortex using µECoG grids and laminar polytrodes. This allows detailed topographic mapping of sensory-evoked neural responses across the cortical surface simultaneously with recordings from multiple neural units distributed across the cortical depth. These protocols enable experiments that characterize distributed cortical activity and may contribute to understanding and eventual interventions for diverse neurological disorders.
Wprowadzenie
Brain functions underlying sensation, cognition, and action are organized and distributed across vast spatial and temporal scales, ranging from the spikes of single neurons to the electrical fields generated by populations of neurons in a cortical column to the topographic organization of columns across brain areas (e.g., somatotopy in somatosensory cortex, tonotopy in primary auditory cortex). Understanding brain function requires sensing electrical signals across these spatial scales1. Neuroscience currently has many widely used methods for monitoring the activity of the brain. Electrophysiologically, laminar polytrodes (such as Neuropixels) enable monitoring of a modest number (~300) of single neurons, typically within a handful of distantly spaced columns, with high (≥1 kHz) temporal resolution. Ca2+ imaging enables monitoring of modest to large numbers of genetically and anatomically identified single neurons within ~1-2 mm spatial extent at a lower (~10 Hz) temporal resolution2. fMRI enables monitoring the metabolic state of large numbers of neurons (~1 M neurons in a 36 mm3 volume) across the entire brain at very low (~0.2 Hz) temporal resolution. EEG/MEG enables monitoring of electrical activity from the whole cortical surface/brain at modest temporal resolution (<100 Hz) and very low spatial resolution (centimeters)3. While each of these methodologies has provided fundamental, synergistic insights into brain function, methods that enable direct sensing of electrophysiological signals at high temporal resolution from precise anatomical locations across broad spatial regions of the cortex are nascent. The need for broad spatial coverage is emphasized by the fact that in the brain, neuronal function changes much more dramatically across the surface compared to the depth4.
Electrocorticography (ECoG) is a method in which grids of low-impedance electrodes are implanted onto the surface of the brain and allow for recording or stimulation of the cortex1,5. ECoG is typically deployed in human neurosurgical settings as part of the clinical work-up for treating pharmacologically intractable epilepsy. However, it also provides unique insights into distributed cortical processing in humans, such as speech and sensory topographic mapping6,7. These capabilities have motivated its use in animal models, including monkeys, rats, and mice5,8,9,10,11. In rodents, it has recently been shown that micro-ECoG (µECoG) enables high temporal resolution (~100 Hz) direct electrical monitoring of neuronal populations with columnar spatial resolution (~200 µm) and broad spatial coverage (many millimeters). µECoG enables researchers to investigate distributed neural dynamics associated with complex sensory processing, cognitive functions, and motor behaviors in animal models12,13. Recent advances have integrated µECoG with optogenetics and laminar polytrode recordings14,15,16,17,18,19,20, allowing for multiscale investigations of cortical networks and bridging the gap between micro-scale neuronal activity and macro-scale cortical dynamics21,22. Critically, because the µECoG signal is very similar in humans and non-human animal models, the use of µECoG makes translation of results and findings from animal models to humans much more direct23. As such, integrative approaches are crucial for advancing our understanding of neural circuitry and hold promise for developing novel therapeutic interventions for neurological disorders5,24,25.
Consequently, there is an emerging need for protocols that integrate high-density µECoG arrays with laminar recordings and optogenetic tools to enable comprehensive multiscale investigations of cortical processing8,26. To address this gap, we have developed custom-designed µECoG devices featuring 128 low-impedance electrodes with 40 µm electrode diameter and 20 µm inter-electrode spacing on a flexible, transparent polymer substrate (parylene-C and polyimide) with perforations between electrodes, enabling simultaneous µECoG and laminar polytrode recordings with optogenetic manipulations13,22. Key aspects of this experimental protocol include: (i) columnar spatial resolution and large-scale coverage of cortical activity through high-density µECoG arrays; (ii) the ability to record from multiple cortical layers using laminar polytrodes inserted through the µECoG grid; and (iii) the incorporation of optogenetic techniques to selectively activate or inhibit specific neuronal populations, thus enabling causal dissection of neural circuits27,28,29. The high-density configuration allows for high spatial resolution recordings, effectively providing a "columnar view" of cortical activity, as previous studies have shown that µECoG signals can resolve activity at a spatial scale comparable to the diameter of the rodent cortical column (~20 µm)11. This integrated methodology allows for simultaneous multiscale monitoring and manipulation of neural activity, potentially enabling causal experiments to determine the neuronal sources of µECoG signals as well as distributed cortical processing. To achieve these objectives, this manuscript provides detailed protocols for the use of high-density µECoG arrays in two combinations.
First, we describe µECoG combined with the manipulation of layer 5 (L5) pyramidal cells in the mouse primary somatosensory cortex (S1). In the mouse, the µECoG array is placed epidurally (due to the surgical intractability of durotomy in mice). An optic fiber is positioned over the grid or combined with a lens to focus the optogenetic light over a small target area of the cortical surface. The optogenetic strategy is described here for inhibition of layer 5 excitatory neurons but can be readily adapted to any population of neurons provided with the corresponding, population-specific, Cre-expressing mouse line. Second, we describe the combined use of µECoG with silicon laminar polytrodes to simultaneously record cortical surface electrical potentials (CSEPs) and single-unit spiking activity from multiple neurons across cortical layers from rat auditory cortex (A1). The array has perforations between electrodes, enabling the insertion of multichannel laminar polytrodes through the grid to record neuronal activity across different cortical layers. During the craniotomy procedure, the µECoG array is placed subdurally over the auditory cortex, and the laminar polytrode is inserted through the perforations. Neural signals from the µECoG and laminar probe are recorded simultaneously, sampled at 6 kHz and 24 kHz, respectively, using an amplifier system optically connected to a digital signal processor.
Protokół
Both protocols follow the same key steps (anesthesia, fixation, craniotomy, µECoG recording) but have notable differences. In the following description, the shared steps are merged, while the specificities of each protocol are annotated. These steps below correspond to µECoG recording with optogenetics (Mouse) or µECoG recording with a laminar probe (Rat). All procedures described here were conducted in compliance with the local ethical or legal authorities (IACUC or Ethics Committees). Medications used may vary according to the approved ethical protocol.
1. Preparation and protocol for mouse and rat procedures
- Notable differences between mouse and rat protocols
- Setups for surgery versus electrophysiological recordings
- For a rat, use the same setup during both surgery and electrophysiological recordings.
- For a mouse, perform the surgery in the first setup and electrophysiological recordings in the second setup.
- Head fixation
- For a rat, use the same snout clamp for surgery and electrophysiological recording.
- For a mouse, use a snout clamp for surgery and an external metal headbar in the setup for electrophysiology to allow fixation under light isoflurane anesthesia. Implant the headbar with dental cement on the skull.
- Recording setup: Use distinct acquisition electronics, recording software, and sensory stimulation software for the two species.
- For the mouse protocol, utilize the SpikeGadgets system (https://spikegadgets.com) and the open-source Trodes software (https://spikegadgets.com/trodes/) for data acquisition.
- For the rat protocol, utilize the recording software Synapse - Neurophysiology Suite (https://www.tdt.com/component/synapse-software/) for data acquisition.
- Induce anesthesia by injection (Rat) or inhalation (Mouse).
- Recording location
- Conduct recordings in the somatosensory cortex (S1) for a mouse.
- Conduct recordings in the primary auditory cortex (A1) for a rat.
NOTE: This difference in anatomical localization requires different craniotomy sites for each species.
- Setups for surgery versus electrophysiological recordings
- Preparing and testing the grid
- Soak the grid (excluding the connector board) in a diluted enzymatic detergent (50% detergent, 50% distilled water) for at least 1 h.
- Transfer it to a bath of pure distilled water and allow it to air-dry in a safe, clean location.
- Perform platinum black electrodeposition as part of the initial preparation of the µECoG device, not before every recording session.
NOTE: Once deposited, the Platinum Black coating forms a stable layer that remains effective for multiple recordings, though its performance should be monitored through regular impedance testing. Platinum Black electrodeposition (target range of 10-20 kΩ at 1 kHz.) decreases electrode impedance and improves signal-to-noise ratio in neural recordings. - To perform the electrodeposition, prepare a chloroplatinic acid solution (typically 1-3% Chloroplatinic acid [H2PtCl6]) containing a small amount of lead acetate (around 0.005%) as a deposition modifier. Connect the µECoG electrodes to serve as the working electrode in a three-electrode electrochemical cell, with a platinum counter electrode and Ag/AgCl reference electrode.
- Apply a constant current density of approximately -0.5--2 mA/cm² for 10-30 s while monitoring impedance values.
- Test and record the impedance of grid electrodes (e.g., with a Nano-Z).
- Check the grid over a light source and prepare the grid for use in post-surgery recording.
- Reference cable soldering
- For a mouse, solder the end of a silver wire (10 mm long, 30 G) to a gold pin for connecting to the reference lead of the recording system.
2. Surgery
- Preparation of materials and general monitoring (Animal care and recording)
- Preparation: Clean and disinfect the surgical area thoroughly with an appropriate disinfectant. Ensure all surgical instruments are sterilized, typically using an autoclave.
- Surgical tool placement: Arrange surgical tools on the sterile surgical pad. Stock the surgical area with cotton-tip applicators and absorbent cotton surgical triangles. Dispose of any biohazardous waste in a dedicated disposal bag.
- Temperature regulation: Turn on the heating pad within the surgical and electrophysiological recording site. Control the heating pad's temperature throughout the surgery and recording.
- Surgical pad: Place a blue surgical pad or blanket over the temperature-regulation bed.
NOTE: This pad should feature a soft cotton white bottom, which should face upwards. - Microscope positioning: Prepare the microscope and the attached illuminator (e.g., LED ring) off to one side of the surgical area. Check that it is functioning properly.
- Surgical drill: Prepare the surgical drill for the craniotomy procedure.
- Oxygen supply: Set the oxygen tank flow rate to 1.0 L/min (Rat) or 0.5 L/min (Mouse) and place the oxygen mask near the regulation pad. The animal will need continuous oxygen following anesthesia.
- Fluid replacement: Throughout the surgery, replace any fluid loss-aiming for a replacement of at least 1.5% of the animal's body weight (Mouse) or 1 mL per h (Rat). Prepare isotonic solutions accordingly.
- Animal: Bring the animal from the animal facility to the surgery room according to approved procedures. Use mice aged 8 to 16 weeks, either male or female, of C57Bl6 background; likewise, use rats aged 7 weeks old, males, of the Sprague Dawley strain.
- Preparation of medications: Weigh the animal using a scale with 0.1 g precision. Prepare the adequate drug quantities for the surgery, using pre-diluted solutions if necessary.
- Anesthesia induction
- Anesthesia induction for a mouse
- Place the animal in the isoflurane induction chamber (3-5% Isoflurane in 0.5 L/min O2).
- Once deep anesthesia is confirmed (absence of reflex in tail/toe pinch), place the animal on the surgery heating pad and headfix it.
- Inject subcutaneous drugs for general analgesia: Meloxicam: 5 mg/kg and Buprenorphine: 0.1 mg/kg.
- Headfix the mouse following steps 2.2.1.5-2.2.1.6.
- Place the snout in the anesthesia mask and the head loosely in the head mount. To place the mouse in the bite bar, first, ensure that the tongue is below the rod and not between the rod and the roof of the mouth. Use forceps to move the tongue if necessary.
- Insert the animal's incisors into the hole on the rod of the bite bar. Secure the mouse anesthesia mask (1.5-2% Isoflurane in 0.5 L/min O2) by gently tightening the fixation screw. Head stabilization during surgery is ensured solely by the bite bar.
- Protect the animal's eyes with a petroleum-based eye ointment or lubricant to prevent drying during surgery.
- Maintain anesthesia throughout the procedure with a continuous flow of isoflurane through the mask.
- Anesthesia induction for a rat
- Use isoflurane initially to sedate the animal to ease the injection of induction anesthesia.
- Administer the drugs for anesthesia and analgesia:
Meloxicam: Dosage of 5 mg/kg, concentration 10 mg/mL, 0.4 mL/kg
Ketamine: Dosage of 90 mg/kg, concentration 100 mg/mL, 0.9 mL/kg
Xylazine: Dosage of 10 mg/kg, concentration 100 mg/mL, 0.1 mL/kg - Allow the animal to reach deep anesthesia within 15-30 min, depending on weight and age.
- Anesthesia monitoring
- Continuously monitor the animal's vitals (respiratory rate) throughout the procedure. Check the respiratory rate as a particularly useful sign of early changes in the anesthetic state, and adjust the anesthetic level if the respiratory rate changes.
- The paw withdrawal reflex is a critical sign of the anesthetic state. Test this reflex periodically, as its total absence ensures sufficient levels of anesthesia for surgery.
- Anesthesia induction for a mouse
- Head fixation and monitoring of vital signs
- Animal vitals monitoring
- Check and record the animal's vital signs on an experimental sheet. If the animal's reflexes (e.g., paw withdrawal) are not fully extinguished, administer an additional half-dose of supplementary ketamine (Rat) or increase the isoflurane concentration by increments of 0.5% (Mouse).
- Head fixation for a rat
- Once the rat is fully anesthetized (no paw or tail reflexes), insert the animal's incisors into the hole on the rod of the head mount.
- Carefully insert the points of the mounting arms into the ridge of the nose to fix the head during surgery, ensuring that it does not make contact with the eyes.
- Adjust the angle of the arms until the roof of the animal's mouth is firmly pressed against the rod. Ensure the skull remains immobile under pressure.
- Secure both arms of the mount by tightening the screws with a hex wrench.
- Oxygen setup for a rat
- Secure the plastic tubing from the oxygen tank over the animal's muzzle and nose, fastening it with surgical tape. Avoid creases in the tubing that may obstruct airflow. Set the oxygen tank to a flow rate of 1 L/min.
NOTE: Animal vitals, including heart rate and respiratory rate, should be checked at 15-30 min intervals throughout the procedure.
- Secure the plastic tubing from the oxygen tank over the animal's muzzle and nose, fastening it with surgical tape. Avoid creases in the tubing that may obstruct airflow. Set the oxygen tank to a flow rate of 1 L/min.
- Animal vitals monitoring
- Scalp incision
- Shaving and preparation
- Shave the area from the upper snout to the back of the head, extending from one eye to the other and around the ears. Remove the bulk of the fur with scissors or an electric clipper, and then apply depilatory cream.
- Disinfection
- Disinfect the area using a cotton swab soaked in Betadine, then rinse with a cotton swab soaked in 70% ethanol. Repeat this process three times and finish with a final Betadine application to ensure the area is sterile.
- Local anesthetic Injection
- Inject the local anesthetic Lidocaine (1%, 0.1 mL for mouse/0.4 mL per kg for rat) subcutaneously into the midline of the animal's scalp. Gently massage the scalp to spread the lidocaine, and wait for 5 min to allow the anesthetic to take effect.
- Incision
- For a mouse, lift a point on the skin with tweezers and resect a small section of skin (approximately 1 cm in diameter) using surgical scissors.
- For a rat, make a precise incision on the anterior side of the scalp, just above the nose, at the midline using the scalpel. Gently pull back the skin, creating a straight incision from between the eyes to the base of the skull. Carefully lift the scalp, cut away connective tissue, and expose the skull fully.
- Expose the craniotomy site following steps 2.4.4.4-2.4.4.5.
- With a scraper, clear away connective tissue and periosteum on the top of the skull. Flush saline and use aspiration or a surgical sponge to clean the site.
- Use surgical clips on the skin margins to facilitate clear exposure of the skull region where the craniotomy will be performed.
- Shaving and preparation
- Craniotomy
- General drilling procedure
- Set surgical drill speed to a low setting of 5000 rpm or 7000 rpm for experienced surgeons. Do all drilling while visualizing through the microscope.
- Hold the drill parallel to the surface of the skull and rest gently against the surface.
- With light pressure on the pedal, begin drilling in a single location. Perform drilling in short intervals (5-10 s) with frequent checks for changes in bone color.
NOTE: The bone will begin an opaque white, and as the hole becomes deeper, it will become more translucent, revealing a pink tint. - When the drilling has gotten close to the brain, slow down and look for signs of moisture seeping into the hole. When the hole is dark pink and has a slight shine, stop drilling. Using a short 30 G needle, gently puncture the remaining layer of bone. Clear liquid should well out of the new hole.
- Drilling procedure for a mouse
- To place a reference electrode for physiological recordings, drill a burr hole in the frontal part of the hemisphere ipsilateral to the recorded area.
- Define the contour of the craniotomy by drilling a shallow trench on its perimeter. In the medio-lateral axis, start from the lateral bone ridge as a reference, and trace a 4 mm window.
- In the antero-posterior axis, drill a 3 mm window starting ~ 1mm anterior of the posterior bone ridge. The final open craniotomy size is approximately a 4 x 3 mm window.
- Drilling procedure for a rat
- Drill two holes: one in the left posterior quadrant, the other in the right anterior quadrant.
- Inject masseter muscle with a second dose of lidocaine (0.4 mL/kg at 10 mg/mL), and distribute evenly where the cut is to be made.
- Resect only the minimal set of muscles needed to expose the craniotomy area.
- Using a fresh #10 scalpel blade, create a transverse dorsal-ventral cut in the bundle of muscle above the animal's jaw (right side). Hold the posterior edge of the cut with gripping forceps and peel away from the skull while cutting along the bony ridge of the cheekbone. In this way, the muscle can be detached from the bone with minimal bleeding.
- Resect the anterior muscle in a similar way until a fissure line in the skull is revealed. This line will be the anterior boundary of the craniotomy window.
- Clear muscle from around the posterior ridge with the scalpel and forceps, using a strong light source to avoid cutting into highly vascularized regions.
- Grind down the posterior ridge using the drill until it is no longer raised above the skull's surface.
NOTE: This step is essential for setting down the µECoG grid to make direct contact with the cortical surface. - Drill the dorsal edge of the window just above the ridge where the resected muscle was attached. Place the posterior edge anterior to the drilled-down posterior ridge. Place the anterior edge posterior to the fissure line extending down near the eye socket.
NOTE: When clearing the anterior muscle, care must be taken to avoid the eye.
- General drilling procedure
- Craniotomy window drilling
- Drilling the craniotomy window (Tips)
- When drilling, ensure that the drill bit is kept parallel to the skull surface. Apply as little force as possible, using the drill like a brush by allowing the drill to make light contact with the skull while using short, repetitive motions along the intended craniotomy line.
NOTE: In rats, the posterior edge of the window has the thickest bone. If drilling too far back, the bone may exhibit a flaky, "crunchy" quality that complicates gauging drilling progress. If placed incorrectly, this bone area may reveal veiny red coloration that gives a false impression of proximity to the brain. - Drill each side of the craniotomy window until the bone appears pale pink with a thin white fissure or crack running along its length. Apply light pressure; the bone should produce a distinct "wiggle" when fully drilled. If the crack appears disjointed, continue drilling lightly until achieving a continuous line.
- When drilling, ensure that the drill bit is kept parallel to the skull surface. Apply as little force as possible, using the drill like a brush by allowing the drill to make light contact with the skull while using short, repetitive motions along the intended craniotomy line.
- Removing the thinned skull within the craniotomy window
- When the skull has been thinned enough that extremely light pressure causes the whole window to wiggle visibly, remove the thinned skull.
- Flush the craniotomy site with a drop of saline and wait at least 1 min. This weakens the thinned bone and helps the bone to detach from the dura. Drain excess saline with an absorbent cotton triangle or vacuum.
- Carefully lift the thinned skull using forceps, avoiding damage to the underlying tissue.
- Use a hemostatic sponge to keep the brain moist.
- Tightly grip the window on the dorsal and ventral sides with toothed forceps and pull directly away from the skull. If there is any difficulty pulling the window out of the site, stop and resume light drilling until the bone is sufficiently weakened.
- For mouse µECoG recordings, leave the dura intact.
- Drilling the craniotomy window (Tips)
- Cement and headpost implant for a mouse
- Insert and secure the reference wire.
- Insert the silver wire end ~1 mm into the burr hole, enough to contact the surface of the brain but not to cause bleeding.
- Apply the dental cement in place while applying the first layer.
- Preparation of dental cement
- Use a cooled ceramic mixing dish to prepare the dental cement mixture. This cement thickens quickly and requires the regular preparation of a new mixture. Wipe clean the mixing dish before preparing a new mixture.
NOTE: The cement should never be in direct contact with the brain.
- Use a cooled ceramic mixing dish to prepare the dental cement mixture. This cement thickens quickly and requires the regular preparation of a new mixture. Wipe clean the mixing dish before preparing a new mixture.
- Application of first layer
- Apply the first layer of cement around the craniotomy and across the entire skull using micro applicators. This layer acts as electrical insulation between the skull and the metal headbar.
- Completely surround the craniotomy with cement, including lateral coverage, to provide adequate protection for theopen craniotomy and µECoG grid.
- Positioning the metal headbar
- Attach the large section ofthe headbar to its holder without fully tightening it.
- Position the headbar as desired, laying the thin section along the skull midline in contact with the cement surface.
- Securing the implant
- Cover the headbar with dental cement and connect it to the cement surface.
- Removing the holder
- Allow a few minutes for the dental cement to strengthen.
- Once the headbar is fully secured, remove it by first removing the screw from the holder. Then, retract the holder backward, ensuring no force is applied to the headbar.
- Insert and secure the reference wire.
- Durotomy for rat surgery
NOTE: This is a challenging surgical step.- Lifting the dura
- Using No. 5 forceps, held as parallel to the brain's surface as possible, lift a small portion of the dura away from the brain.
- Use a fresh 30 G needle (as short as possible) to carefully tear the lifted dura.
NOTE: The dura mater is a thin, transparent layer of tissue that lies directly on top of the brain. It is removed for µECoG recordings in rats. It is critical to perform the durotomy without disturbing the vasculature on the brain's surface. Recommended methods for performing the durotomy include using forceps and a syringe needle to puncture the dura before pulling it back or using a duratome tool anchored near the skull to retract the dura carefully.
- Resection of the dura
- Continue gripping the dura with the forceps and lifting it away from the brain. Create a diagonal tear with the needle while lifting.
- Use the forceps to carefully peel the dura towards the sides of the craniotomy window, ensuring the brain surface remains undisturbed.
- Lifting the dura
- Transferring the mouse to the setup for electrophysiological recording
- Remove the animal from the surgery setup by gently lifting the snout and incisors from the incisor bar and then pulling back the animal. Inject Chlorprothixene (1 mg/kg, intraperitoneal [IP]), a sedative that enables continuous anesthesia to be maintained using a lower Isoflurane concentration.
- Place the mouse in the electrophysiological recording setup.
- Make sure that the heating pad is in place and working properly.
- Head fix the animal using the headbar on the holder in the electrophysiological setup.
- Bring the isoflurane mask close to fully cover the animal's snout.
- Anesthesia adjustment
- Gradually reduce anesthesia levels to 0.7-1% Isoflurane (in increments of 0.5% maximum every 5 min).
- Monitor the animal's respiratory rate and movements.
NOTE: The respiratory rate should increase slightly compared to the surgical state, but the animal should not be moving. - If the animal is moving, immediately increase the isoflurane concentration to 2% before slowly returning it to a lower level in increments of 0.5%.
- Inserting whiskers for sensory stimulation
- Attach the mouse's vibrissae to the whisker stimulation device. In this protocol, insert nine vibrissae into short 10 µL pipette tips, which are connected to piezoelectric actuators that provide rapid deflections of the vibrissae.
3. Recording
- Installing the grid
- Preliminary steps
- Turn on the recording system and amplifier.
- Check the animal's vital signs.
- Procedure
- For positioning the animal and tools, follow steps 3.1.2.2-3.1.2.4.
- Place the animal in the recording setup, and ensure the craniotomy remains moist by regularly applying a saline solution.
- For a rat, position the micromanipulator on the rig's railing, located well behind the craniotomy site, to avoid interference.
- For a mouse, place the micromanipulator laterally to the craniotomy site alongside the animal.
- To attach and position the grid on a mouse, follow steps 3.1.2.6-3.1.2.12.
- Attach the µECoG grid to the headstage using the ZIF-clip connectors (headstage connector). Hold the headstage's electronic board in place via a mechanical bar fixed to a micromanipulator.
- Lower the µECoG grid horizontally to align flat over the craniotomy along the anteroposterior axis.
NOTE: Along the lateral-medial axis, the grid's edge should be near the craniotomy's medial border. - Once the grid is positioned near the brain but not in contact with it, attach the grid's reference wire to the implanted silver wire-gold pin. If required, attach the ground wire to the animal (e.g., to an uncovered muscle) to reduce electrical noise.
- Further, lower the grid to contact the brain.
- Move the grid laterally to "glide" over the moist dura surface. Continue adjusting until the grid is centered along the mediolateral axis.
- Use aspiration or a surgical sponge around the craniotomy's edges to remove any excess saline solution.
- Once the preparation is slightly drier, ensure the grid adheres more firmly to the dura and does not slide over its surface. When drier, apply a lateral to medial movement to the flexible grid, ensuring contact with the most lateral electrodes. The grid's flexible cable will naturally bend to match the brain's contour.
- To position the grid on a rat, follow steps 3.1.2.14-3.1.2.18.
- Secure the stem of the holding fork in the micromanipulator, ensuring that the connector board of the grid will hover over against the posterior side of the craniotomy window when lowered.
- Adjust the position of the micromanipulator on the railing so that the grid is roughly above the craniotomy site. Lower the grid until it hovers close above the brain surface. Moisten the brain surface with a small drop of saline.
- Conduct these steps using the microscope. Using the dials of the micromanipulator, adjust the grid position until it lies flat against the brain surface in the center of the craniotomy.
- Wick away moisture carefully using an absorbent cotton triangle without touching the grid itself. Ensure every row of the grid is in contact with the brain surface.
NOTE: Removing moisture prevents the passive spread of the electrical signal through the fluid between the cortical surface and the grid, which spatially diffuses the signal sensed at the electrode. - Using number 2 or number 5 forceps, insert the grid grounding wire into the same burr hole or insert the reference wire into a burr hole and the ground wire into nearby muscle tissue.
NOTE: Wires should be inserted only ~1 mm, enough to contact the brain but not to cause bleeding or trauma to the brain.
- Preliminary steps
- Checking the positioning of the grid
- Monitoring electrophysiological activity
- Observe the electrophysiological activity using the recording software. Under light anesthesia, brain signals are variable and can exhibit a variety of patterns.
- Proper connection of the grid, reference, and ground wires should yield a high signal-to-noise ratio, with signal amplitudes in the range of mV. Monitor noise in the high-frequency range using bandpass filtering with Trodes (e.g., 100-6000 Hz) and ensure it does not exceed a few tens of microvolts (µV).
- Assess the sensory responsiveness using noise (e.g., clapping or snapping fingers) to induce visible event-related cortical surface electrical potentials (CSEPs).
NOTE: Stimulation of a single whisker should evoke a clear, sharp event-related CSEP in only a few channels (Mouse). - Grid position verification
- For a rat, confirm that the grid is correctly positioned over the auditory cortex. The first block recorded should typically be a 60-s white noise stimulus set to verify that the grid registers a proper response from the brain. Conduct white noise and tone diagnostic recordings with the grid only before inserting polytrode to help determine whether the grid was placed correctly and if there is a signal response.
- For a mouse, to verify grid positioning, perform a quick mapping session with 20-30 whisker deflections spaced by 350 ms. Record the activity in the local field potential (LFP) band using Trodes and analyze it offline with a custom MATLAB code to visualize the spatial extent of whisker-evoked activity.
- Repositioning
- If the grid requires adjustment, moisten the cortical surface with drops of saline over the grid.
- Leave the saline for 30 s to 1 min before attempting to lift the grid.
- Carefully and slowly lift the grid.
- Reposition it using the steps described in step 3.1.
- Monitoring electrophysiological activity
- Laminar polytrodes for a rat
- Polytrode setup
- First, connect the headstage adapter to the back side of the polytrode. Clip the connector to the third set of channels onto the board of the adapter. Ensure the black mark on the clip faces the right side of the business end of the polytrode.
- Polytrode Insertion
- Insert the polytrode into the brain until the very last (topmost) electrodes are visible above the cortical surface. A slow descent (down to 1 µm/s) improves the signal quality. Wait for 15 min, allowing the brain to adjust to the polytrode's presence.
- After 15 min, check whether the last electrodes have entered the cortical surface. If not, lower the polytrode slightly more and wait an additional 10 min before proceeding.
- Polytrode setup
- Positioning the optogenetic light source on a mouse
- Use either a fine adjustment screw system in three dimensions, mounted on an articulated arm, or a micromanipulator to mount the optical fiber holder.
- To guide the light source and aid in positioning the fiber, turn on the optogenetic light at a low intensity. Use the articulated arm to coarsely position the optogenetic light toward the target area.
- Focus and fine-tune the fiber's position using either a micromanipulator or fine adjustment screws.
- Recording the signals
- Preparation
- Unplug all unnecessary lights, extension cords, and surge protectors in the surgical rig to reduce electrical interference. Turn off the overhead lights in the rig.
- Close the door to the isolated recording space and the door to the surgery room before commencing the experiment.
- Starting acquisition
- For a rat, start Synapse on the recording platform/computer and confirm that acquisition is functional by previewing and checking for signals. Elicit large, sharp voltage transients in the µECoG signal by presenting stimuli near the animal, i.e., clapping.
- For a mouse, start the Recording Session in Trodes.
- Hydration
- Inject the rat or mouse subcutaneously with respectively 1 mL or 0.1 mL of saline every 1-2 h during recording to prevent dehydration. For a rat, wait 5-10 min after administering saline before running a new recording block.
- Stimulus sets
- For a rat, once the recording site is confirmed, proceed with recording the required stimulus sets. An example set might include
White Noise (60 s)
Tone Diagnostic (5 min)
Pure Tone (23 min)
Dynamic Moving Ripple
Tone 150 (15 min)
TIMIT (38 min) - For a rat, re-present white noise and tone diagnostics any time the grid is repositioned.
- Tactile stimuli for a mouse: Provide tactile stimuli in a trial structure, with each trial containing a train of random whisker deflections every 350 ms. In the provided example, each trial includes 14 deflections presented over 4500 ms.
- Optogenetic stimuli for a mouse: In some trials, apply a square pulse of optogenetic light over the entire trial duration (5 s). Determine the required light level based on the opsin used and on the depth of tissue to be reached using estimates of light penetrance (https://web.stanford.edu/group/dlab/cgi-bin/graph/chart.php)
- For a rat, once the recording site is confirmed, proceed with recording the required stimulus sets. An example set might include
- Preparation
- Cleanup
- Lifting and cleaning the grid
- Once recording has finished, close the recording software and replug light sources in the rig.
- If the brain is dry, apply a small drop of saline onto the brain surface using a syringe. Leave the saline for 30 s to 1 min before attempting to lift the grid.
- Working under the microscope, gently lift the grid from the brain surface using micromanipulators.
- If additional force is needed while lifting the grid, use carbon-tipped forceps (closed) to gently lift the grid from the brain. Ensure the movement of the micromanipulator is slightly anterior to gently peel the grid away from the brain surface.
- Once the grid has been fully removed, detach it from the gripping fork and clean it following steps 3.6.1.6-3.6.1.7.
- Soak the grid (excluding the connector board) in a diluted enzymatic detergent (50% Enzol, 50% distilled water) for at least 1 h. Afterward, transfer it to a second bath of pure distilled water and allow it to air-dry in a safe, clean location.
- If areas of the grid have deposited blood or tissue, use a cotton triangle soaked in enzymatic solution to gently wipe it clean.
- Once dry, return the grid to its box.
- Euthanizing the animal
- For a mouse, remove the animal from head-fixation and place it in the euthanasia chamber. Add a gauze with 5 mL isoflurane and wait 60 s after cessation of respiration. Verify lack of withdrawal reflex and decapitate using sharp scissors.
- For a rat, inject 0.2 mL of pentobarbital IP. Wait for 60 s after cessation of respiration, lie the animal on its back, and use a #11 blade to perform a double thoracotomy.
- Cleaning the equipment
- Take all surgical tools to the lab sink and lay them on a surgical towel. Spray the tools with 10% bleach solution and wash thoroughly in the sink. For dirtier tools, allow them to soak in a bleach solution before washing.
- Alternatively, use a powder detergent (e.g., Contrex AP) with water by scrubbing the instruments with a brush in the sink.
- Once tools are clean and rinsed, wipe them down with alcohol wipes and return them totheir storage space.
- Sanitizing the workspace
- Dispose of all used needles and blades in the sharps container.
- Dispose of contaminated cotton swabs, triangles, and alcohol wipes in the biohazard bag.
- Wipe all work surfaces in the rig room with alcohol and clean all instruments before closing the workspace.
- Lifting and cleaning the grid
Wyniki
We have described the protocols for recording electrocorticographic signals combined with optogenetic methods and laminar recordings. Here, typical signals obtained from the somatosensory cortex of the mouse (Figure 1, Figure 2, and Figure 3) and within the auditory cortex of rats in response to sensory stimulation (Figure 4, Figure 5, and Figure 6) are presented.
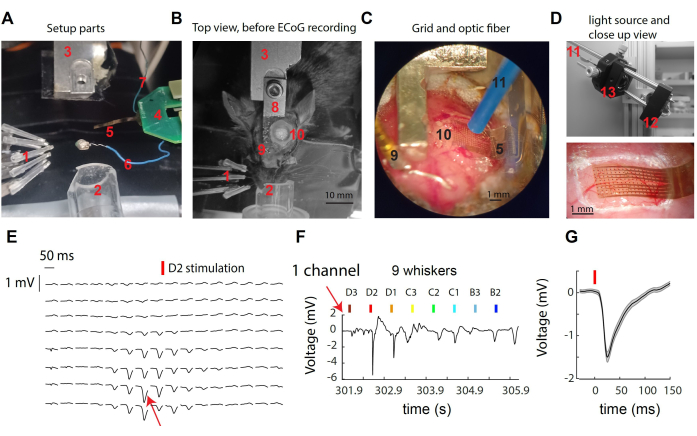
Figure 1: Recording µECoG signals over the mouse whisker somatosensory cortex. (A) Components of the setup. See text. (B) Top view of the surgical setup before µECoG recording, showing placement of the head restraint and craniotomy site. (C) Detailed view of the µECoG grid and optic fiber positioned on the exposed cortex. (D) Top: Light source is located outside of the recording box. Light from a 473 nm LED is collected in the optical fiber through a lens tandem. Bottom: close-up of the cortical grid over the mouse cortex in an example recording. (E) Representative µECoG traces from all 16x8 channels showing trial averaged responses to whisker D2 stimulation. (F) Raw voltage trace from the single channel indicated in (E) across single deflections of multiple whiskers. The channel exhibited the strongest response during stimulation of the whisker D2. (G) The average evoked response across trials in the single channel (E,F), which exhibited a sharp voltage deflection following whisker stimulation. Please click here to view a larger version of this figure.
Figure 1A-D provides images of various components of the system used for recording and for optogenetics manipulation in the mouse S1 cortex. Labeled elements include: 1- nine independent whisker stimulator in a 3 x 3 array, 2- nose cone for isoflurane anesthesia, 3- head-post holder/counterpart, 4- µECoG back-end PCB, connected to the headstage PCB with a double ZIF connector; 5- µECoG grid of electrodes at the tip of an 8 mm flexible cable; 6- reference wire; 7- ground wire; 8- headpost implant; 9- reference gold pin (insulated from the headpost with dental cement); 10- craniotomy on the left hemisphere, over the S1 whisker barrel cortex; 11- 1 mm diameter optogenetic fiber (held by a micromanipulator, not shown); 12- a star-shaped LED driven by an LED driver (not shown); 13- light collection through a tandem of aspheric lenses. When stimulating a single whisker, a rapid deflection of the surface potential at a small number of electrodes (Figure 1E) is observed. This cluster of electrodes represents the local signal that peaks in the cortical column of the stimulated whisker30. Looking at a single electrode, we observe the strongest response to stimulation of its preferred whisker and the weaker or no response to stimulation of more distant whiskers (Figure 1F). In this example, the onset of the deflection occurs around 10 ms after whisker vibration, with an average amplitude of 1 mV (Figure 1G).
Figure 2 presents example recordings during optogenetic inhibition. The light can reach the cortical tissue through the transparent substrate of the grid (Figure 2A). However, when using a large-diameter fiber or any spatially broad source of light, photons also hit the electrodes, producing an opto-electric artifact (Figure 2B). In this protocol of inhibition, we used a 5 s long square pulse of light. The resulting opto-electric artifacts are only present at the onset and offset of the light. In an animal without opsin, whisker stimulation presented during the light on does not evoke a different response from the whisker stimulation presented in trials with the light off (Figure 2C). In contrast, light stimulation of the inhibitory opsin in a subpopulation of excitatory neurons leads to a decrease in the amplitude of the sensory-evoked response (Figure 2D)
Figure 2C,D present examples of optogenetic suppression in the time-frequency domain. To analyze the µECoG data, we first apply a common average referencing (CAR) to remove signals that are undifferentiated across electrodes (e.g., breathing) followed by a Morse wavelet transform, which reveals active frequency bands over time. Neural activity in the frequency domain typically exhibits an approximate power law of 1/f2,3. To reveal the sensory evoked signal more uniformly across the frequency domain, we apply Z-scoring separately for each frequency band. The Z-scoring is based on the statistics of the signal during baseline epochs. Here, we use a time window that precedes the stimulus, -3000 ms to -1000 ms prior to the trial start. This process yields the stimulus-evoked Z-score for each frequency band (Figure 2C,D).
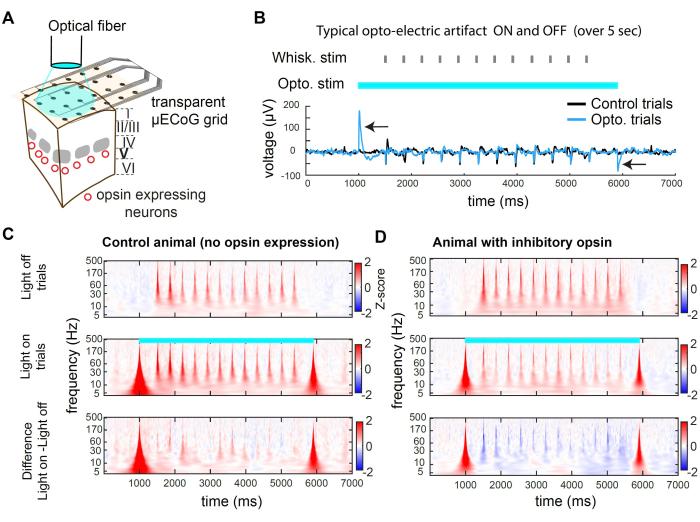
Figure 2: Optogenetic suppression in wS1 during µECoG recording in mice. (A) Schematic of a representative optogenetic experiment. An optical fiber is directly pointed at the brain, allowing transient inhibition of the neuronal population expressing an inhibitory opsin. (B) Voltage trace recorded in the channel at the center of optogenetic stimulation, averaged across trials. The black arrow indicates opto-electric artifacts at the onset and offset of the 473 nm light. Note the response to whisker deflection (random whisker identity) in the middle of the trial. The artifact is transient and does not affect the recording of sensory-evoked activity after a delay (here 500 ms). (C) Average spectrogram of an example channel in light-off trials, light-on trials, and the difference between the two, in an animal where no opsin was expressed. Note that the opto-electric effect induces a broadband, transient artifact, and the whisker-evoked response is unaffected by the light. (D) Example trial-averaged spectrogram across light-off trials, light-on trials, and the difference between the two in an Rbp4-Cre mouse32 in which st-GtACR2.0 was expressed in excitatory neurons of layer 5. Note the suppression of whisker evoked response in the spectrogram. Please click here to view a larger version of this figure.
Figure 3 presents easy-to-implement variations in the light delivery system. Using a smaller optical fiber, or a simple lens, it is possible to target a specific area of the cortex15. The light coming out of the fiber is rapidly diverging and reaches cortical tissue beyond the targeted area. By incorporating an aspheric lens (f =16 mm) at the optical fiber output, it is possible to focus the light to a smaller surface area (Figure 3A,C), down to roughly the diameter of a single cortical column (Figure 3C). Light is also less diverging within the cortex. Ideally using a laser or a powerful source of light with a tandem set of lenses, it is possible to target a single cortical column with a collimated beam of light. However, it is important to consider that light will scatter within the tissue, which may partially illuminate neighboring columns. The light artifact measured in the µECoG data will reveal where light has been delivered over the cortical surface; see the comparison of Figure 3B versus Figure 3D. The light artifact is measured as the peak power in a high-frequency range (65-500 Hz) 5 ms after light onset.
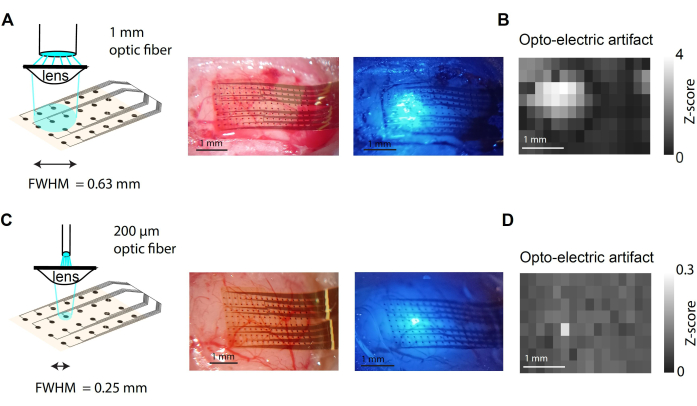
Figure 3: Light delivery to focal points of the cortex. (A) Light from the 1 mm diameter optical fiber is focused on the brain surface. This simple adjustment allows for more precise targeting of light on the brain surface and within the tissue. (B) The spatial extent of the light delivered to the brain is measured from the opto-electric artifact. (C,D) The setting is as in panels A,B with a 200 µm optical fiber, which allows it to directly target an area of 250 µm diameter, roughly the size of a cortical column. Please click here to view a larger version of this figure.
Rat µECoG and Laminar Polytrode recordings
Figure 4A-D provides images of various components of the system used for rat primary auditory cortex recordings. Specifically, labeled elements include: (1) µECoG micromanipulator, (2) rodent stereotaxic rig, (3) µECoG cable withZIF-clip connectors, (4) laminar polytrode micromanipulator, (5) rat nose cone and head stabilization apparatus, (6) electrostatic speaker for stimulus presentation, (7) laminar polytrode cable withZIF-clip adapter, (8) µECoGZIF-clip attached by a two-pronged fork, (9) 32-channel polytrode device, (10) electrostatic speaker, (11) alligator clips to hold surgical site open, (12) grounding wires, one inserted into the cortex through the burr holes in the left posterior quadrant or right anterior quadrant and another is grounded to tissue, (13) close-up view of µECoG grid, (14) close-up view of inserted polytrode. Figure 4E displays the average neural spectrogram derived from the recorded electrical potentials of a single µECoG electrode in response to 50 ms tone pips of the same frequency and attenuation (N = 20 trials). Across frequencies, the evoked response exhibits a sharp peak between 25 ms and 30 ms (indicated by red solid lines) after the stimulus onset (left gray dotted line). The apparent response preceding the stimulus onset (ostensibly acausal) is due to the large bandwidth at lower frequencies of the constant-Q transform, which smooths (acausal, but no phase offset) rather than filters (causal, but induces a phase offset) the signal. Therefore, the time of the peak response remains accurate. At the time of the peak evoked response, we observed that the z-scored cortical surface electrical potential (CSEP) was multimodal across frequencies. Specifically, it exhibited three primary non-harmonic peaks: the first and largest in the 40-180 Hz gamma/high-gamma (γ/Hγ) range, the second in the 200-450 Hz ultrahigh-gamma (uHγ) range, and a final peak above 500 Hz associated with multi-unit activity (MUA) (Figure 4F)11. This stimulus-evoked multimodal structure is robust across all tuned electrodes. Here, we focus on Hγ because of its prevalence in human electrocorticography (ECoG) recordings31. In Figure 4G, we display a µECoG array that was placed subdurally alongside a silicon laminar polytrode to simultaneously measure cortical surface electrical potentials (CSEPs) and spiking activity across cortical laminae. The custom-fabricated µECoG array consisted of 8×16 electrodes with a 20 µm pitch and 4 µm contact diameter, and we utilized a 32-channel laminar polytrode configured as 2 × 16 channels with a 4 µm pitch and 1 µm contact diameter. The µECoG array was sufficiently large to cover the entire rat primary auditory cortex (A1), and its small 4 µm diameter electrodes enabled the measurement of local CSEPs suitable for deriving auditory tuning. The spatial resolution of the µECoG high-gamma (Hγ) signal is ~20 µm, comparable to the radius of a rat cortical column. Thus, µECoG provides a "columnar view" of cortical activity. Perforations in the µECoG grid allowed the laminar polytrode to pass between surface contacts, enabling direct recording of neuronal activity across cortical laminae (Figure 4H). Example voltage traces recorded from µECoG and laminar polytrode electrodes are shown in Figure 4I.

Figure 4: Recording µECoG and laminar polytrode signals over the rat primary auditory cortex (A1). (A) Components of the µECoG and laminar polytrode setup. See text. (B) Top view of the surgical setup before µECoG and laminar polytrode recording, showing placement of the head restraint and craniotomy site. (C) Detailed view of the µECoG grid and laminar polytrode on the exposed auditory cortex.(D) A close-up view of the µECoG grid placement on the cortex, including size of individual electrodes (40 µm) and between electrodes (200 µm).(E) Average z-scored wavelet decomposition of a single-channel response to a single frequency-attenuation pair. The vertical red lines indicate the peak frequency response period shown in F.(F) Red- Average peak frequency response across 20 presentations of a single frequency-attenuation pair. Gray- Standard error. The frequency axis is on a log scale. Canonical neural frequency bands are indicated across the top.(G) Photomicrograph of an 8 16 µECoG grid on the surface of rat primary auditory cortex (A1). A 32-channel laminar polytrode has been inserted in the center µECoG window. (H) Schematic of 3D multiscale recording of cortical activity. (I) Top: Spectrogram of 50 ms pure tone pips. Middle: In red, the average cortical surface electrical potential of 4 µECoG electrodes (gray). Bottom: 32-channel laminar polytrode voltage traces are arranged by cortical depth Please click here to view a larger version of this figure.
Figure 5 displays the recording of µECoG signals simultaneously with a spiking activity using a laminar silicon polytrode (Camb64). The µECoG signal, recorded from a surface electrode, is shown as a raw voltage trace (Figure 5A) and its wavelet transform, highlighting frequency decomposition over time (Figure 5B). Multi-unit activity was recorded using the polytrode, as illustrated by a representative raw voltage trace from a single channel (Figure 5C), where individual action potentials (spikes) were detected using a simple thresholding method (voltage crossing a -120 µV threshold). The extracted spike waveforms from this channel are well-defined for the majority of spikes, although they could originate from multiple sources of neurons (Figure 5D, inset). Across multiple channels in the same penetration, the average spike waveforms further confirm the consistency of well-defined spiking activity recordings (Figure 5E). Thus, these recording methods could support single-unit recording with spike sorting analysis and analysis of quality metrics such as the interspike interval or firing rates (Figure 5F). In summary, the quality of the laminar probe recordings is good, with clear waveform morphology on single channels. These results illustrate that this method enables the simultaneous acquisition of µECoG and spiking activity.

Figure 5: Simultaneous recordings of µECoG and columnar spiking activity. (A) Example raw voltage trace from a single µECoG channel.(B) Wavelet transform of the same µECoG channel, showing frequency decomposition over time. (C) Example raw voltage trace from a single polytrode channel (acute 64-channels silicon probe camb64), showing action potentials (spikes) detected using a simple voltage threshold. (D) Extracted spike waveforms from the polytrode channel in (C) aligned and color-coded based on the distribution of voltage amplitude. 7 out of 142 spike waveforms are not shown, as they were considered outliers based on their distance from the main cluster in spike width and amplitude (see inset). (E) Average multi-unit spike waveforms extracted from 9 channels in the same penetration. (F) Interspike interval (ISI) histograms for different polytrode channels (e.g., Channels 21, 27, 63, and 61), with corresponding firing rates (FR) indicated in Hz. ISI violations (< 5ms) represented less than 5% of spikes in all units. Please click here to view a larger version of this figure.
With the capability to perform simultaneous recordings using the custom-designed µECoG array and laminar polytrode, we investigated how µECoG signals compare to neural unit recordings across cortical depth in terms of auditory tuning. Figure 6A presents Frequency-Response Amplitude (FRA) plots, which depict the high-gamma (Hγ) response as a function of auditory stimulus frequency and amplitude. The top panel shows FRAs from a 2 × 16 subset of µECoG electrodes positioned on the auditory cortex, while the bottom panel displays FRAs from a 1×16 subset of laminar polytrode electrodes inserted through the µECoG array's perforations (indicated by gray arrows). Remarkably, the FRAs obtained from the µECoG electrodes closely resemble those from the laminar polytrode recordings, suggesting that µECoG signals are similarly tuned to neural unit activity across cortical layers.
Leveraging the high spatial resolution of the µECoG array, we generated a high-resolution tonotopic map of multiple auditory cortical fields based on the Hγ activity11. In Figure 6B, each electrode's best frequency is color-coded, revealing the tonotopic organization across the cortical surface. The 8 × 16 µECoG array covered several auditory cortical fields-including the primary auditory cortex (A1), posterior auditory field (PAF), and ventral auditory field (VAF)-with approximate boundaries demarcated by black lines. This detailed mapping underscores the µECoG array's capability to provide a "columnar view" of cortical activity, capturing functional organization with a spatial resolution of less than 20 µm, comparable to the dimensions of a rat cortical column. These findings demonstrate that µECoG recordings not only reflect the tuning properties observed in neural unit recordings across cortical depth but also enable high-resolution mapping of functional cortical organization.
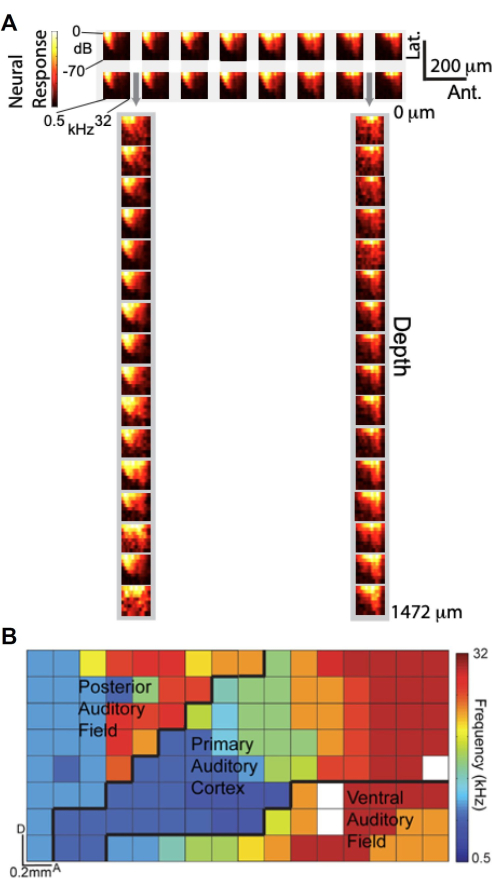
Figure 6. µECoG signals are similarly tuned to neural unit spike recordings across the depth and reveal the tonotopic organization of the auditory cortex. (A) Frequency-Response Amplitude plots (FRAs), which display the high-gamma response (color bar) as a function of the frequency (x-axis) and amplitude (y-axis) of the auditory stimulus. (top) FRAs from 2 16 subset of µECoG electrodes on the auditory cortex; (bottom) FRAs from 1 16 subset of laminar polytrode electrodes inserted through perforations in the µECoG array, indicated by gray arrows. (B) High-resolution tonotopic organization of multiple auditory cortical fields derived from high-gamma activity. Each pixel is color-coded according to the best frequency of that electrode. The 8x16 µECoG array displayed here covers multiple auditory cortical fields (A1, PAF, and VAF), and the approximate boundaries are demarcated (black lines). Please click here to view a larger version of this figure.
Dyskusje
The protocols described here enable integrating high-density micro-electrocorticography (µECoG) arrays with laminar probes and optogenetic techniques. The ease of use of this protocol in rodent models makes it a powerful tool for the investigation of cortical dynamics, and the number of subjects can be easily increased. The high-density µECoG grid allows for efficient, spatially precise mapping of cortical topography across multiple areas in mice and rats, leveraging the critical role of topographical representations in brain organization33. The addition of laminar recording allows for the examination of cortical dynamics across multiple spatial and temporal scales. The inclusion of optogenetics enables causal manipulation to determine the relationships between specific neural populations and their contributions to cortical surface-evoked potentials (CSEPs) and cortical processing34.
Optogenetics allows for the selective modulation of specific neuronal populations, enabling the investigation of their causal roles in generating cortical activity patterns and participating in computation whose signatures can be sensed with µECoG. For example, our studies demonstrate that targeting a specific cell type can alter cortical surface electrical potentials (CSEPs). This approach could be used to dissect the neural cell type basis of µECoG signals, allowing us to identify which neuronal cell types contribute to characteristic sensory-evoked activities, such as those observed in the high-gamma band. Other large-scale phenomena, such as cortical rhythms in different frequency bands21, or spatially organized activity, including traveling waves35,36, could similarly be investigated. Additionally, a range of genetically modified mouse lines and opsins are readily available, providing opportunities to explore specific circuit mechanisms. Optogenetic techniques could be employed to examine the functional effects of horizontal connections between columns37, which play a crucial role in various sensory computations, such as surround suppression38 or perceptual binding39. In summary, the ability to manipulate neuronal activity through optogenetics enables testing the links between specific neuronal populations and the properties of CSEPs or between population and specific cortical computations measurable with µECoG. This approach could effectively dissect the relationships between local neural structures and global cortical activity.
Laminar polytrode recordings enable sampling of single-neuron activity from multiple neurons within small cortical volumes, i.e., within an individual cortical column. These recordings are crucial because individual neurons can independently encode distinct information, employing selective coding of stimuli-such as the "Jennifer Aniston neurons" described by Quiroga et al.40 -- or providing complementary representations in a higher-dimensional space, as seen in mixed selectivity41. Traditionally, electrophysiologists studied neural activity in the context of simple, parametrically designed stimuli and behaviors (e.g., single whisker deflection or pure tones, as deployed here). The representation of such stimuli tends to be fairly spatially localized (e.g., individual columns). However, many ethologically relevant stimuli and behaviors are more complex, and as such, typical patterns of neural activity during such paradigms frequently extend beyond single columns - even all across the brain42. In these instances, µECoG offers a comprehensive readout that captures high-temporal resolution, columnar resolved activity across multiple columns simultaneously. In summary, the protocol described here effectively bridges the gap between local processing within individual cortical columns and the more extensive dynamics that occur across multiple columns in an entire cortical area and across areas.
As general guidance and troubleshooting, we propose a few recommendations. Conventional acute in vivo electrophysiological protocols typically advise keeping the brain wet during exposure as a means of prolonging the health of the exposed brain. While this is likely useful in some circumstances, our experience suggests the opposite is true for µECoG recordings in rodents. Indeed, we found that the recording quality was qualitatively better when the µECoG arrays were placed on a slightly dry cortical surface. We believe this arises because having saline, a highly conductive ionic solution positioned between the cortical surface and the recording electrodes, homogenizes electrical signals generated by the brain. Effectively, saline is 'shorting' the electrodes together. Because µECoG grids monitor the continuous electrical field generated by populations of neurons, it is important to ensure that electrophysiological systems are well-assembled and denoised. During recordings, the connection with the reference electrode is critical. Without improvement during the recording, the pre-processing of the data can incorporate a notch filter at 50 Hz or 60 Hz to remove line noise. However, this will dramatically alter the signal and so must be accounted for in subsequent analysis. Considering optogenetic light stimulation, the opto-electrical artifact must be minimized or at least accounted for in the experimental design (e.g., by including a delay following onset or offset of light). Ideally, light is delivered in the area between the electrodes using a small optical fiber diameter, or focusing or collimating the light. If this artifact cannot be completely avoided (but see studies proposing different probe designs15,20,43 including clear electrodes), it can be reduced by using the least amount of light necessary. New generation opsins require less light for effectiveness44,45. We suggest calibrating the optogenetic light power prior to the experiment using laminar probes. The artifact shape can also be modified and reduced by avoiding sharp transitions in the light stimulus (e.g., using a light ramp instead of a square pulse). In any case, control conditions using animals that do not express opsins are advisable to differentiate genuine changes in neural activity from artifact-related signals.Finally, the electrodeposition process creates a rough, high surface area coating that enhances charge transfer between electrode and tissue while maintaining mechanical stability during recordings, reducing electrode impedance by 1-2 orders of magnitude compared to bare platinum and enabling better neural signal detection46.
Acute µECoG offers flexibility and reduced complexity in experimental setups, allowing for detailed mapping of neural activity over the course of tens of minutes of recordings. As a mesoscale method, it enables an inter-areal but not whole-brain tracking of cortical activity, though the exact sources of the signals remain uncertain1,47. In the future, multimodal studies should provide a better picture of the signal's origin. Acute µECoG is limited in capturing long-term neural dynamics and may be influenced by transient factors such as surgical recovery or anesthesia48,49. In contrast, chronic µECoG enables prolonged observation of neural activity, providing insights into processes like learning, plasticity, and disease progression10,13. Chronic µECoG also presents challenges such as electrode stability, potential signal degradation, and risks associated with long-term implantation, including tissue scarring or infection50,51. These challenges tend to be less severe when compared to penetrating electrodes and are supposedly further reduced with epidural implantation of the µECoG in mice (at the cost of lower signal quality)52. It is possible to reuse the same µECoG grid across many sessions in the same or different animals, removing the grid at the end of a session and replacing it in the next session. In this sub-acute configuration, the brain must be kept moist at all times and protected with a glass coverslip between sessions. We have observed that µECoG grids are fairly durable; an individual grid can be reused ~20 times with proper handling and cleaning, making them cost-effective recording devices. Grids can be designed with diverse numbers and geometries of electrode layouts. The results here suggest that reducing electrode pitch to <200 µm results in only negligible improvements in functional resolution due to the granularity of neural representations in the underlying cortex.
Ujawnienia
The authors declare no competing financial interests.
Podziękowania
This work was supported by Lawrence Berkeley National Laboratory LDRD for the Neural Systems and Machine Learning Lab (K.E.B.), NINDSR01 NS118648A (K.E.B.& D.E.F.), and NINDS R01 NS092367 (D.E.F.).
Materiały
| Name | Company | Catalog Number | Comments |
| 1 disposable #11 blade | Swann Morton | 303 | For surgical procedures |
| 2 disposable #10 blades | Swann Morton | 3901 | For surgical procedures |
| 30 mm cage bars | Thorlabs | ER | cage components |
| 30 mm cage plate | Thorlabs | CP33T | holding the lenses |
| 70% ethanol | Decon Labs | V1016 | Cleaning / Disinfectant (diluted to 70%) |
| Amalgambond PLUS Adjustable Precision Applicator Brush Teal 200/Bx | Henry Schein | 1869563 | precision applicator for the cement |
| Amalgambond PLUS Catalyst 0.7 mL Syringe Ea | Henry Schein | 1861119 | cement component |
| Amplifier (Tucker-Davis Technologies) | Tucker-Davis Technologies | PZ5M-512 | Used for auditory stimulus and recording software. |
| Articulated arm | Noga | DG60103 | for holding the fine adjustment screw system |
| Aspheric lenses for light collection (and one for focusing the light) | Thorlabs | ACL25416U-B | for collecting LED light |
| Auditory equipment | Tucker-Davis Technologies, Sony, Cortera | RP2.1 Enhanced Real-Time Processor/HB7 Headphone Drive | Used for auditory stimulus and recording software. |
| Buprenorphine | Sterile Products LLC | #42023017905 | General analgesia |
| C&B Metabond Base Cement Ea | Henry Schein | 1864477 | cement component |
| C&B Metabond L-Powder Cement Clear 3 g | Henry Schein | 1861068 | cement component |
| Chlorprothixene hydrochloride (mouse) | Sigma Aldrich | Cat. No. C1671 | For sedation, must be prepared the same day and kept at 4 |
| Custom-designed 128-channel micro-electrocorticography (μECoG) grids | Neuronexus | E128-200-8-40-HZ64 | For neurophysiology recordings. Placed onto the cortex. |
| Dengofoam gelatin sponges | Dengen dental | 600034 (SKU) | can be used dry or wet, saturated with sterile sodium chloride solution |
| Drill bit, size 5 to 9 (Mouse) | Fine Science Tools | 19007-XX | XX is the size of the drill bit e.g. 05 or 09. For mouse procedures |
| Drill bitSteel Round Bur (5.5 mm/7.5 mm) | LZQ Tools | Dental Bar Drill Bit Stainless Steel Bur | For rat procedures |
| Dumont No. 5 forceps | Fine Science Tools | 11251-10 | For surgical procedures |
| Dumont tweezers #5 bent 45° | World precision instruments | 14101 | for removing craniotomy window |
| DVD Player (Sony) | Sony | CDP-C345 | System used to accept and play back stimulus sets |
| Electrostatic Speaker | Sony | XS-162ES | Used for auditory stimulus and recording software. Located within the rig, plays sound to the sedated rodent |
| Enzymatic detergent (Enzol) | Advanced sterilization products | 2252 | Cleaning/Disinfectant |
| EverEdge 2.0 Scaler Sickle Double End H6/H7 #9 | Henry Schein | 6011862 | for scrubing the skull |
| Fine adjustment screw system in 3 dimension | Narishige | U-3C | for precise positioning of the optical fiber end |
| Gold pin | Harwin Inc | G125-1020005 | Used for contact reference in mouse Soldered to the silver wire |
| Gripping forceps | Fine Science Tools | 00632-11 | For surgical procedures |
| Isoflurane | Covetrus | 11695067772 | require a vaporizer |
| Ketamine (Hydrochloride Injection) (Rat) | Dechra | 17033-101-10 | Anesthesia/Analgesic |
| LED | New Energy | LED XLAMP XPE2 BLUE STARBOARD | Blue LED light source |
| LED driver | Thorlabs | LEDD1B | LED driver |
| Lidocaine | Covetrus | VINB-0024-6800 | to be diluted to 1% in saline |
| Meloxicam | Covetrus | 6451603845 | Anti-inflammatory used for general analgesia |
| Micromanipulator | Narishige (Stereotaxic Rig) | SR-6R + SR-10R-HT components | Used to manipulate ECoG and rodent with fine movements |
| No. 2 forceps | Fine Science Tools | 91117-10 | For surgical procedures |
| No. 55 forceps | Fine Science Tools | 1129551 | For surgical procedures |
| Ophtalmic lubricant (Artificial tears) | Akorn | 17478-062-35 | Used to protect eyes from dessication during surgical procedures |
| Optical fiber 200µm Core diameter | Thorlabs | M133L02 | FC/PC connector 2 m long |
| Pentobarbital (Rat) | Covetrus / Dechra | VINV-C0II-0008 | Anesthesia/Analgesic |
| Platinum Black | Sigma | 205915-250MG | For neurophysiology recordings (Used for electroplating the contacts on the μECoG grids). |
| Povidone Iodine 10% | Betadine | https://betadine.com/medical-professionals/betadine-solution/ | no catalog number ( not retail ) |
| Powder detergent (Contrex AP) | Decon Labs | 5204 | Cleaning / Disinfectant |
| Pre-cut tape for oxygen tube | ULINE (Various Providers) | S-14726 | Used to attach oxygen tube to the nose-cone of the rodent stereotaxic rig |
| Scalpel handle # 3 | World precision instruments | 500236-G | for blades # 10, #11 and #15 |
| Scraper | Fine Science Tools | 1007516 | For surgical procedures |
| Short 30 G needles | ExelInt | 26437 | For surgical procedures and injections |
| Silver Wire | Warner Instruments | 63-1319 | For neurophysiology recordings (Used for grounding and as a reference electrode). |
| Sterilized saline (0.9% sodium chloride for injection) | Hospira | 00409-7101-67 (NDC) | For dilution of injectable, and replacement of body fluids |
| Stoelting Hopkins Bulldog | Fine Science Tools | 10-000-481 | For surgical procedures |
| Surface disinfectant (Coverage Plus NDP Disinfectant) | Steris life science | 638708 | Cleaning/Disinfectant |
| TDT ZIF-clip connectors for acquisition. | Tucker-Davis Technologies | ZIF-Clip Analog Headstages | Connects ECoG with outside acquisition equipement |
| Two-pronged holding fork | Tucker-Davis Technologies | Z-ROD128 | Used to connect the TDT-clips with the micromanipulator |
| Xylazine (Rat) | Covetrus | 1XYL006 | Anesthesia/Analgesic |
Odniesienia
- Buzsáki, G., Anastassiou, C. A., Koch, C. The origin of extracellular fields and currents-EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 13 (6), 407-420 (2012).
- Chen, T. -. W., et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 499 (7458), 295-300 (2013).
- Nunez, P. L., Srinivasan, R. . Electric Fields of the Brain: The Neurophysics of EEG. , (2006).
- Mountcastle, V. B., Powell, T. P. Central nervous mechanisms subserving position sense and kinesthesis. Bull Johns Hopkins Hosp. 105, 173-200 (1959).
- Khodagholy, D., et al. NeuroGrid: recording action potentials from the surface of the brain. Nat Neurosci. 18 (2), 310-315 (2015).
- Lewis, C. M., Bosman, C. A., Womelsdorf, T., Fries, P. Stimulus-induced visual cortical networks are recapitulated by spontaneous local and interareal synchronization. Proc Natl Acad Sci U S A. 113 (5), E606-E615 (2016).
- Bouchard, K. E., Mesgarani, N., Johnson, K., Chang, E. F. Functional organization of human sensorimotor cortex for speech articulation. Nature. 495 (7441), 327-332 (2013).
- Ledochowitsch, P., et al. Fabrication and testing of a large area, high density, parylene MEMS µECoG array. , (2011).
- Bosman, C. A., et al. Attentional stimulus selection through selective synchronization between monkey visual areas. Neuron. 75 (5), 875-888 (2012).
- Rubehn, B., Bosman, C., Oostenveld, R., Fries, P., Stieglitz, T. A MEMS-based flexible multichannel ECoG-electrode array. J Neural Eng. 6 (3), 036003 (2009).
- Baratham, V. L., et al. Columnar localization and laminar origin of cortical surface electrical potentials. J Neurosci. 42 (18), 3733-3748 (2022).
- Kellis, S., et al. Decoding spoken words using local field potentials recorded from the cortical surface. J Neural Eng. 7 (5), 056007 (2010).
- Viventi, J., et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat Neurosci. 14 (12), 1599-1605 (2011).
- Ledochowitsch, P., Olivero, E., Blanche, T., Maharbiz, M. M. A transparent µECoG array for simultaneous recording and optogenetic stimulation. , (2011).
- Ledochowitsch, P., et al. Strategies for optical control and simultaneous electrical readout of extended cortical circuits. J Neurosci Methods. 256, 220-231 (2015).
- Dougherty, M. E., Nguyen, A. P. Q., Baratham, V. L., Bouchard, K. E. Laminar origin of evoked ECoG highgamma activity. Annu Int Conf IEEE Eng Med Biol Soc. 2019, 4391-4394 (2019).
- Tian, H., Xu, K., Zou, L., Fang, Y. Multimodal neural probes for combined optogenetics and electrophysiology. iScience. 25 (1), 103612 (2022).
- Gonzales, D. L., et al. A translaminar spacetime code supports touchevoked traveling waves. bioRxiv. , (2024).
- Leonard, M. K., et al. Largescale singleneuron speech sound encoding across the depth of human cortex. Nature. 626 (7999), 593-602 (2024).
- Renz, A. F., et al. OptoEDura: a soft, stretchable ECoG array for multimodal, multiscale neuroscience. Adv Healthc Mater. 9 (17), 2000814 (2020).
- Buzsáki, G. . Rhythms of the brain. , (2006).
- Yang, W., et al. A fully transparent, flexible PEDOT:PSS-ITO-Ag-ITO based microelectrode array for ECoG recording. Lab Chip. 21 (6), 1096-1108 (2021).
- Buzsáki, G. Largescale recording of neuronal ensembles. Nat Neurosci. 7 (5), 446-451 (2004).
- Schalk, G., et al. Realtime detection of eventrelated brain activity. Neuroimage. 43 (2), 245-249 (2008).
- Kellis, S., et al. Multiscale analysis of neural activity in humans: implications for microscale electrocorticography. Clin Neurophysiol. 127 (1), 591-601 (2016).
- Buzsáki, G., Schomburg, E. W. What does gamma coherence tell us about interregional neural communication. Nat Neurosci. 18 (4), 484-489 (2015).
- Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G., Deisseroth, K. Millisecondtimescale, genetically targeted optical control of neural activity. Nat Neurosci. 8 (9), 1263-1268 (2005).
- Cardin, J. A., et al. Driving fastspiking cells induces gamma rhythm and controls sensory responses. Nature. 459 (7247), 663-667 (2009).
- Kwon, S. E., Yang, H., Minamisawa, G., O'Connor, D. H. Sensory and decisionrelated activity propagate in a cortical feedback loop during touch perception. Nat Neurosci. 19 (9), 1243-1249 (2016).
- Petersen, C. C. The functional organization of the barrel cortex. Neuron. 56 (2), 339-355 (2007).
- Crone, N. E., Sinai, A., Korzeniewska, A. Highfrequency gamma oscillations and human brain mapping with electrocorticography. Prog Brain Res. 159, 275-295 (2006).
- Gerfen, C. R., Paletzki, R., Heintz, N. GENSAT BAC crerecombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron. 80 (6), 1368-1383 (2013).
- Mountcastle, V. The columnar organization of the neocortex. Brain. 120 (4), 701-722 (1997).
- Deisseroth, K. Optogenetics. Nat Methods. 8 (1), 26-29 (2011).
- Muller, L., Chavane, F., Reynolds, J., Sejnowski, T. J. Cortical travelling waves: mechanisms and computational principles. Nat Rev Neurosci. 19 (5), 255-268 (2018).
- Sato, T. K., Nauhaus, I., Carandini, M. Traveling waves in visual cortex. Neuron. 75 (2), 218-229 (2012).
- Gilbert, C. D., Wiesel, T. N. Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. J Neurosci. 9 (7), 2432-2442 (1989).
- Angelucci, A., et al. Circuits and mechanisms for surround modulation in visual cortex. Annu Rev Neurosci. 40 (1), 425-451 (2017).
- Singer, W., Gray, C. M. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci. 18, 555-586 (1995).
- Quiroga, R. Q., et al. Invariant visual representation by single neurons in the human brain. Nature. 435 (7045), 1102-1107 (2005).
- Fusi, S., Miller, E. K., Rigotti, M. Why neurons mix: high dimensionality for higher cognition. Curr Opin Neurobiol. 37, 66-74 (2016).
- Stringer, C., et al. Spontaneous behaviors drive multidimensional, brainwide activity. Science. 364 (6437), eaav7893 (2019).
- Cardin, J. A., et al. Targeted optogenetic stimulation and recording of neurons in vivo using celltypespecific expression of channelrhodopsin2. Nat Protoc. 5 (2), 247-254 (2010).
- Mardinly, A. R., et al. Precise multimodal optical control of neural ensemble activity. Nat Neurosci. 21 (6), 881-893 (2018).
- Mahn, M., et al. Highefficiency optogenetic silencing with somatargeted anionconducting channelrhodopsins. Nat Commun. 9 (1), 4125 (2018).
- Ferguson, J. E., Boldt, C., Redish, A. D. Creating lowimpedance tetrodes by electroplating with additives. Sens Actuators A Phys. 156 (2), 388-393 (2009).
- Halnes, G., et al. . Electric Brain Signals: Foundations and Applications of Biophysical Modeling. , (2024).
- Franks, N. P. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 9 (5), 370-386 (2008).
- Alkire, M. T., Hudetz, A. G., Tononi, G. Consciousness and anesthesia. Science. 322 (5903), 876-880 (2008).
- Schalk, G., Leuthardt, E. C. Braincomputer interfaces using electrocorticographic signals. IEEE Rev Biomed Eng. 4, 140-154 (2011).
- Chestek, C. A., et al. Longterm stability of neural prosthetic control signals from silicon cortical arrays in rhesus macaque motor cortex. J Neural Eng. 8 (4), 045005 (2011).
- Branco, M. P., et al. Nine decades of electrocorticography: a comparison between epidural and subdural recordings. Eur J Neurosci. 57 (8), 1260-1288 (2023).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone