Method Article
Isolation of Embryonic Ventricular Endothelial Cells
W tym Artykule
Podsumowanie
Primary cell culture is a useful technique for analyzing specific populations of cells, particularly from transgenic mouse embryos at specific developmental stages. Herein, embryonic ventricles are dissected and dissociated, and antibody-conjugated beads recognize and separate out the endothelial cells for further analysis.
Streszczenie
Cell culture has greatly enhanced our ability to assess individual populations of cells under myriad culture conditions. While immortalized cell lines offer significant advantages for their ease of use, these cell lines are unavailable for all potential cell types. By isolating primary cells from a specific region of interest, particularly from a transgenic mouse, more nuanced studies can be performed. The basic technique involves dissecting the organ or partial organ of interest (e.g. the heart or a specific region of the heart) and dissociating the organ to single cells. These cells are then incubated with magnetic beads conjugated to an antibody that recognizes the cell type of interest. The cells of interest can then be isolated with the use of a magnet, with a short trypsin incubation dissociating the cells from the beads. These isolated cells can then be cultured and analyzed as desired. This technique was originally designed for adult mouse organs but can be easily scaled down for use with embryonic organs, as demonstrated herein. Because our interest is in the developing coronary vasculature, we wanted to study this population of cells during specific embryonic stages. Thus, the original protocol had to be modified to be compatible with the small size of the embryonic ventricles and the low potential yield of endothelial cells at these developmental stages. Utilizing this scaled-down approach, we have assessed coronary plexus remodeling in transgenic embryonic ventricular endothelial cells.
Wprowadzenie
The advent of immortalized cell lines has revolutionized basic cell biology 1. The currently available cell lines are derived from a wide range of organs and encompass all the major cell types. However, established cell lines have some limitations. The process of immortalization obviously changes the behavior of the cells, specifically with respect to life span and proliferation, but can also affect the expression of unexpected proteins, such as cytoskeletal proteins 2. In addition, although many different cell lines are available, there is significant diversity among even a single cell type within an entire organism. Endothelial cells in particular show diverse behaviors based on whether they line arteries or veins and what kind of flow is present in the vessel 3. Even more pressing from a developmental perspective, however, is that the vast majority of the available cell lines are derived from adult tissue (see, e.g. the collection available via ATCC). These adult cell lines likely do not recapitulate the dynamic nature of their embryonic precursors. The rapidly changing spatiotemporal gene expression patterns observed during development also suggest that an endothelial cell from one organ at a given developmental stage may not behave the same way as an endothelial cell from that same organ at a different developmental stage.
As an alternative to using commercially available immortalized cell lines, primary cells can be isolated from the specific tissue of interest. Among the advantages of this technique, these primary cells can be isolated from a specific organ or even part of an organ at any specific developmental stage. Further, these primary cells can be isolated from the wide variety of available transgenic animals, allowing the in vitro study of gene knockout and knock-in while avoiding other problems such as transfection efficiency. Not surprisingly, many techniques have been published detailing how to isolate specific cell types 4,5. In general, these techniques involve collecting the region of interest, dissociating the cells, tagging the specific cell type of interest, and isolating those cells for further analysis.
To study an early embryonic population of coronary endothelial cells, we scaled down a previously published technique4 for use with a smaller organ. With this scaled-down procedure, we can isolate the coronary endothelial cells from the embryonic heart at specific embryonic stages. These cells can then be used in traditional endothelial assays, such as migration analyses. Until early embryonic cell lines become more prevalent, working with the primary cells is an invaluable technique.
Protokół
1. Prepare Antibody-conjugated Beads
- One day prior to dissection, combine the antibody of choice with the appropriate Dynabeads (e.g. rat IgG Dynabeads for an antibody raised in rat, 4 x 108 beads/ml) per the manufacturer's instructions. For the BD rat anti-CD31 antibody (0.5 μg/μl, used to isolate endothelial cells), 3 μl antibody is added for every 25 μl beads, for a final concentration of 1.5 μg anti-CD31 antibody and 1 x 107 beads/25 μl buffer.
- Incubate beads and antibody overnight at 4 °C.
2. Preparing the Collagen Plate
- Isolated cells form tubules more quickly when grown on a collagen gel as opposed to merely a collagen-coated plate. Therefore, collagen gels are made the night before the dissection, as described in 6. On ice in a tissue culture hood, combine the following reagents (enough for 19 wells): 206.4 μl sterile H2O, 140.4 μl collagen type I (4.08 mg/ml), 38.4 μl 10x M199, and 1.15 μl 5 M NaOH.
- Pipette 20 μl of this collagen gel into the wells of a 384-well tissue culture plate. Place the plate in a 37 °C tissue culture incubator for 30 min.
- Add 80 μl DMEM to each well and incubate for 15 min at 37 °C. Remove the culture medium and repeat twice more.
- After the final rinse, add 80 μl 10% FBS/DMEM to each well and incubate overnight at 37 °C. Remove the culture medium prior to adding the isolated cells.
3. Excise and Digest the Heart
- Euthanize a timed-pregnant mouse at the desired embryonic day using an approved euthanasia technique. All experiments were approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill.
- With the mouse in a supine position, liberally spray the ventral side of the female with 70% ethanol prior to dissection. Using forceps to lift the lower abdominal skin and muscle away from the internal organs, cut open the female's lower abdominal cavity to visualize the internal organs.
- Holding the cervix with forceps, carefully cut caudal to the forceps and begin lifting the uterine horn from the abdominal cavity. While lifting the uterine horn, cut away any connective tissue that holds the horn in place. To finish excising the uterine horn, cut the junction of the uterine horn with the oviduct to free the uterine horn.
- Place the uterine horn in a Petri dish containing cold 1x phosphate-buffered saline (PBS) and rinse as needed. Cut open the uterine horn via the midline to expose the attached embryonic sacs.
- Cut open an embryonic sac at the junction of the placenta and the embryo to avoid accidentally cutting the embryo and retrieve an embryo. Place the embryo in a second Petri dish with cold PBS. Return the Petri dish with the uterine horn to ice.
- Decapitate the embryo to improve access to the chest wall. If genotyping is necessary, cut the tail to remove it and save in an Eppendorf tube placed on ice.
- With the embryo on its back, cut open the chest wall to visualize the heart and lungs. To avoid cutting the heart, make a vertical cut along the side of the rib cage, near a forelimb, followed by a horizontal cut across the bottom of the ribs; then, gently pull the chest wall up and out of the way. Up through approximately embryonic day 13.5, the chest wall is transparent, aiding in visualization.
- Carefully lift the heart using forceps and cut the vessels below. Then, cut above the great vessels to free the heart. If the pulmonary vessels are not cut, the lungs may be removed with the heart. Thus, remove extraneous tissues, like the lungs, if necessary.
- Separate and discard the atria and great vessels from the ventricles. Using scissors, mince the ventricles into small pieces (approximately 1 mm3) and place in approximately 500 μl collagenase (1 mg/ml in sterile PBS). Keep samples on ice until all ventricles have been minced and placed in collagenase.
- Repeat the previous steps until the ventricles have been excised from all embryos, collecting each heart in a separate Eppendorf tube. If all embryos have the same genotype, hearts may be pooled in a single Eppendorf tube. The number of samples processed at a single time may be limited based on the magnet used for isolation; the DynaMag (123-21D) used here holds a maximum of 16 Eppendorf tubes.
- Place the minced ventricles at 37 °C with rocking for 45 min. Every 15 min, gently remove the samples and pipette up and down to mechanically dissociate the cells.
4. Isolating the Endothelial Cells
- Pass the ventricle-collagenase solution through a 70 μm filter to remove remaining clumps of cells.
- Centrifuge the cells at 400 x g for 10 min. Discard the supernatant, replace with approximately 200 μl 10% FBS in DMEM, and pipette up and down to dissociate the cells.
- Repeat the previous step once. Centrifuge the cells at 400 x g for 10 min after the second 10% FBS/DMEM wash. Resuspend the cells in 50 μl 10% FBS/DMEM.
- During the final centrifugation step, prepare the beads.
- Place the Eppendorf tube containing the antibody and beads on a magnet for 1 min.
- With the tube in place on the magnet, remove the supernatant. Remove the tube from the magnet and add approximately 100 μl 10% FBS/DMEM.
- Place the tube on the magnet for 1 min. Remove the supernatant, and wash again with 10% FBS/DMEM. Repeat for a total of 3 washes.
- After the final wash, resuspend the antibody-conjugated beads in 10% FBS/DMEM (5 μl per sample).
- To each cell sample, add 5 μl of the antibody-conjugated beads. Place the bead-cell suspensions at 4 °C with rocking for 30 min.
- In a laminar flow hood, place the Eppendorf tubes containing cells and antibody-conjugated beads on the magnet for 1 min. Remove the supernatant, remove the tubes from the magnet, and wash with approximately 100 μl 10% FBS/DMEM. Repeat for 5 washes.
- After the last wash, place the tubes on the magnet for 1 min, remove the supernatant, and remove the tubes from the magnet. Add approximately 100 μl DMEM.
- Place the tubes on the magnet for 1 min, remove the supernatant, and remove the tubes from the magnet. Add 100-200 μl prewarmed 0.25% trypsin-EDTA. Incubate the samples at 37 °C and 5% CO2 for 5 min.
- Place the tubes on the magnet for 2 min. Carefully transfer the cell-containing supernatant to fresh Eppendorf tubes. Centrifuge for 5 min at 400 x g.
- Remove the supernatant. Resuspend the cells in 80-100 μl growth medium and plate in a 96- or 384-well treated culture dish, depending on the expected yield (approximately 450-600 cells from the ventricles of E14.5-E16.5 embryos). For endothelial cells, use endothelial growth culture medium (defined below).
Wyniki
Using an endothelial-specific antibody that recognizes CD31, endothelial cells were isolated from the ventricles of embryonic (E) 13.5 (Figure 1A) and 18.5 (Figures 1B-1D) embryos. When grown on an untreated culture dish, these cells remain rounded and would form a cobblestone pattern if near confluent (Figure 1A). Dilute collagen has also been used to coat the wells, and while the endothelial cells will adhere to it (Figure 1B), they form fewer chains than when plated on a collagen gel (Figures 1C and 1D). Endothelial cells grown in a well containing a collagen gel (Figures 1C and 1D) form cell-cell interactions and form chains that begin to branch when grown on collagen, and this process occurs faster when grown on the collagen gel compared with the collagen-coated plate. These endothelial chains label positively with the endothelial marker iso-lectin (Figure 1D). The isolated cells survive up to 1 week in culture but have proven resistant to trypsinization (even after 1 hr at 37 °C); thus, they are best suited to terminal experiments.
Endothelial cells isolated from E18.5 ventricles label positively with PECAM (Figure 2A) and Flk1 (Figure 2B). Further, live isolated endothelial cells can be labeled using the endothelial-specific fluorescent dye Syto-16. Figure 2D shows endothelial cells that were isolated from the ventricles of an E14.5 embryo and labeled with Syto-16.
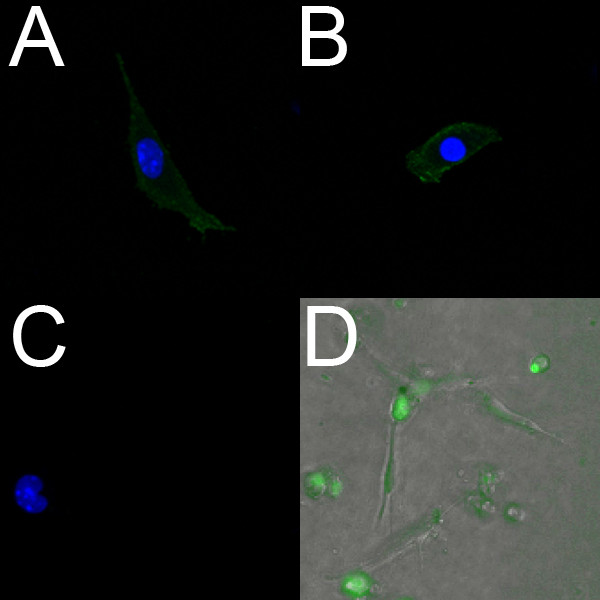
Figure 1. Endothelial cells isolated from embryonic mouse ventricles form chains when grown on a collagen gel. (A) Brightfield 10x image of live isolated ventricular endothelial cells from an E13.5 heart grown on an untreated culture dish; even after 48 hr, cells remain rounded. (B) Brightfield 10x image of fixed isolated ventricular endothelial cells from an E18.5 heart grown on a collagen-coated dish; after 24 hr, some cells have begun elongating (arrow), but most of them remain rounded (arrowhead). (C, D) Confocal images of fixed isolated ventricular endothelial cells from an E18.5 heart grown on a collagen gel, shown in brightfield (C) and labeled with iso-lectin (red). Arrows indicate some of the lectin-positive cells. Scale bars in C-D = 50 μm.
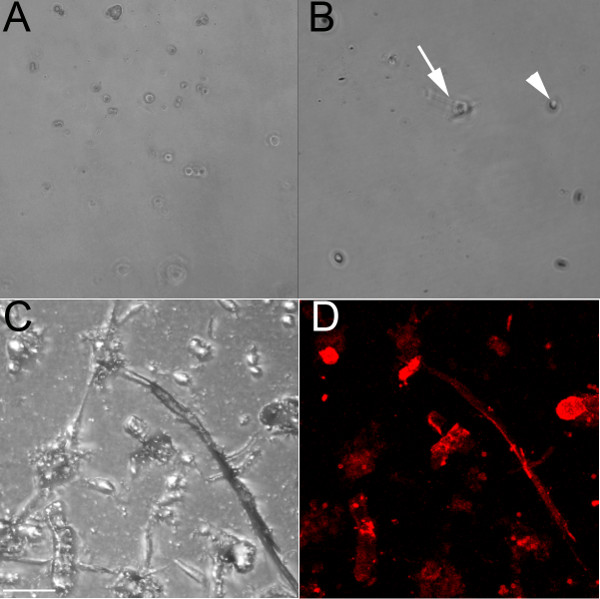
Figure 2. Endothelial cells isolated from embryonic mouse ventricles label positively for endothelial markers. (A-C) Confocal images (40x) of fixed isolated ventricular endothelial cells from an E18.5 heart grown on a collagen-coated dish. These cells label positively for PECAM (A) and Flk-1 (B); the negative control (C) shows no fluorescence. In A-C, the nuclei are labeled with DAPI (blue). (D) Fluorescent overlay (10x) of live isolated ventricular endothelial cells from an E14.5 heart grown on a collagen gel. The cells were incubated with the live endothelial marker Syto-16 (green), and migration was observed using time-lapse microscopy.
Dyskusje
Working with primary embryonic cells allows novel experiments to address in vitro critical steps of development. However, the isolation procedure is not trivial. Critical steps for isolating any type of cells include ensuring that the cells are well separated upon collagenase digestion and then that they are well suspended during the wash steps. Mechanical dissection by pipetting greatly helps separate the cells during the collagenase step, and the filtering step removes clumps of cells. These steps improve the population purity and yield.
The plating density is also a significant concern when isolating cells from a small organ. Because endothelial tube-formation is dependent on the cell density 7, we relied on 384-well tissue culture plates to increase our plating density. Even with this consideration, we have had to modify some analyses to accommodate a lower cell density (Dyer and Patterson, in progress). Thus, if cell number is a concern, a smaller sized culture well may lessen the problem.
Another limitation of isolating primary cells is the specificity of the antibody. Even a recognized endothelial-specific protein such as CD31 is sometimes expressed by fibroblasts 8. If the harvested cell number allows for FAC sorting instead, then the endothelial cells and fibroblasts could be separated by the fluorescence intensity. However, a recent FAC sorting analysis of embryonic endothelial cells required four entire E10.5 embryos to produce enough endothelial cells for mRNA extraction, suggesting that this technique, though powerful, may not be appropriate for smaller organs 9.
Thus, if FAC sorting is not feasible, other techniques can be employed for improving population purity. A two-step selection process, in which the cells are positively selected by one antibody and then negatively selected by failure to bind to a second antibody, is one alternative approach. The fibroblast-specific marker FSP-1 would be one particular candidate 10. Alternatively, the culture medium can be ordered without L-valine, and D-valine can be added instead; fibroblasts are unable to utilize D-valine and thus do not survive 11,12.
Despite these limitations and concerns, studying primary embryonic cell populations allows for a detailed in vitro analysis of developmental processes. This technique can be applied to any organ or region of the embryo and allows a cell type to be compared across different embryonic stages. With the appropriate scaling, even very small populations of primary cells can be obtained and analyzed. These analyses will provide significant insight into how specific subsets of cells behave and change over time.
Ujawnienia
The authors declare that they have no competing financial interests.
Podziękowania
We would like to thank Andrea Portbury for critical reading of the manuscript, the Microscope Service Laboratory of UNC for assistance with the time-lapse imaging, and the NIH (grant # R01HL061656) for funding support.
Materiały
| Name | Company | Catalog Number | Comments |
| REAGENTS | |||
| Timed-pregnant mice | To be dissected at the embryonic stage of interest | ||
| Anti-mouse CD31 | BD Bioscience | 553370 | |
| Rat IgG Dynabeads | Invitrogen | 110-35 | |
| Collagen | BD Bioscience | 354236 | |
| PBS (1x) | |||
| Collagenase, type I | Worthington Biochemical | LS004196 | |
| DMEM | Cellgro | ||
| FBS | Sigma-Aldrich | F2442 | |
| Endothelial cell growth medium | (made in lab as described in notes) | DMEM containing 20% FBS, 5 μM β-mercapt–thanol, 50 μg/ml ECG, and 1x penicillin/streptomycin | |
| β-mercapt–thanol | Sigma-Aldrich | M6250 | |
| ECGS | Biomedical Technologies, Inc. | BT-203 | |
| Penicillin-Streptomycin | Gibco | 15140 | |
| Trypsin-EDTA | Gibco | 25300 | |
| 384-well culture plate | Greiner | T-3037-6 | Plate was prepared with a thin collagen gel, as described in 6; working surface area 10 mm2 |
| Collagen type I | BD | 354236 | Use at a final concentration of 1.5 mg/ml |
| M199, 10x | Invitrogen | 11825-015 | |
| Syto-16 | Invitrogen | S7578 | Used as directed in 13 |
| EQUIPMENT | |||
| Stereoscopic microscope | Nikon | SMZ645 | |
| Cell culture incubator | Thermo | 3110 | |
| DynaMag | Invitrogen | 123-21D | |
| Table-top centrifuge | Thermo | 75002430 | |
Odniesienia
- Landecker, H. . Culturing Life: How Cells Became Technologies. , (2007).
- Kan, C. Y., Wen, V. W., et al. Endothelial cell dysfunction and cytoskeletal changes associated with repression of p16(INK4a) during immortalization. Oncogene. , (2012).
- Dyer, L. A., Patterson, C. Development of the endothelium: an emphasis on heterogeneity. Semin. Thromb. Hemost. 36, 227-235 (2010).
- Dong, Q. G., Bernasconi, S., et al. A general strategy for isolation of endothelial cells from murine tissues. Characterization of two endothelial cell lines from the murine lung and subcutaneous sponge implants. Arteriosclerosis, Thrombosis, and Vascular Biology. 17, 1599-1604 (1997).
- van Beijnum, J. R., Rousch, M., Castermans, K., vander Linden, E., Griffioen, A. W. Isolation of endothelial cells from fresh tissues. Nat. Protoc. 3, 1085-1091 (2008).
- Runyan, R. B., Markwald, R. R. Invasion of mesenchyme into three-dimensional collagen gels: a regional and temporal analysis of interaction in embryonic heart tissue. Dev. Biol. 95, 108-114 (1983).
- Arnaoutova, I., Kleinman, H. K. In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat. Protoc. 5, 628-635 (2010).
- Zeisberg, E. M., Potenta, S. E., Sugimoto, H., Zeisberg, M., Kalluri, R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. Journal of the American Society of Nephrology : JASN. 19, 2282-2287 (2008).
- Chang, L., Noseda, M. Differentiation of vascular smooth muscle cells from local precursors during embryonic and adult arteriogenesis requires Notch signaling. PNAS. 109, 5993-6998 (2012).
- Fehrenbach, M. L., Cao, G., Williams, J. T., Finklestein, J. M., DeLisser, H. M. Isolation of murine lung endothelial cells. American Journal of Physiology: Lung Cellular and Molecular Physiology. 296, L1095-L1103 (2009).
- Frauli, M., Ludwig, H. Inhibition of fibroblast proliferation in a culture of human endometrial stromal cells using a medium containing D-valine. Archives of Gynecology and Obstetrics. , 241-96 (1987).
- Lazzaro, V. A., Walker, R. J., et al. Inhibition of fibroblast proliferation in L-valine reduced selective media. Research communications in chemical pathology and pharmacology. 75, 39-48 (1992).
- Arima, S., Nishiyama, K., et al. Angiogenic morphogenesis driven by dynamic and heterogeneous collective endothelial cell movement. Development. 138, 4763-4776 (2011).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone