Research Article
Development of Mouse Models for Ménétrier's Disease
In This Article
Summary
The current study demonstrates that MT-TGFα mice exhibit spasmolytic polypeptide-expressing metaplasia (SPEM) in the stomach. Leakiness of the promoter prevented the demonstration that chief cell lineages are traced to SPEM. Thus, we additionally developed a doxycycline-inducible mouse model (Doxi-TGFα) and confirmed that SPEM is derived from chief cells.
Abstract
Ménétrier's disease (MD) is a rare acquired premalignant gastric disorder characterized by giant rugal folds, decreased acid secretion, and protein loss. MD patients show increased expression of an EGF receptor (EGFR) ligand, transforming growth factor-α (TGFα) in the stomach. The EGFR-neutralizing antibody, cetuximab, results in rapid clinical improvement and histological remission. Beyond these findings, the etiology and underlying molecular mechanisms are not well understood. The Metallothionein (MT)-TGFα transgenic mouse line is the first MD mouse model that recapitulates histopathological features of MD, including foveolar hyperplasia and loss of parietal cells. In this mouse model, TGFα is driven by the heavy metal-inducible MT enhancer/promoter. Prior studies have used zinc sulfate (ZnSO4) in drinking water or intraperitoneal injections of cadmium sulfate (CdSO4) to induce TGFα. However, we found that MT-TGFα mice develop phenotypes without heavy metal treatment, indicating leakiness of the promoter. We also found that overexpression of TGFα suppresses Mist1 expression, a transcription factor important for chief cell differentiation, thus hindering genetic manipulation in chief cells using the Mist1-CreERT2 mouse line. To overcome this, we developed an inducible mouse model (Doxi-TGFα) in which TGFα is induced by doxycycline treatment (CMV-rtTA; TetO-TGFα). Although the Doxi-TGFα mouse model develops milder phenotypes than the MT-TGFα model, it recapitulated features of MD, including foveolar hyperplasia and loss of parietal cells. Using the Doxi-TGFα mouse model, we found that spasmolytic polypeptide-expressing metaplasia (SPEM) is induced in MD, and SPEM is derived from chief cells by lineage tracing using the Mist1-CreERT2 mouse line. Both MT-TGFα and Doxi-TGFα mouse models offer in vivo models of MD and are useful for investigating the molecular mechanisms underlying MD pathogenesis and treatment options for the disease. The Doxi-TGFα mice will also be a useful model to study the effects of overexpression of TGFα in other tissues.
Introduction
Ménétrier's disease (MD), also known as protein-losing hypertrophic gastropathy, is a rare gastric premalignant condition. Stomachs of MD patients show massive foveolar hyperplasia, which leads to increased gastric mucus secretion, and a decreased number of parietal cells, which leads to decreased gastric acid secretion. In addition, protein is lost selectively across the gastric mucosa, which leads to hypoalbuminemia and peripheral edema1,2,3. The pathogenesis of MD was not known until it was reported that transgenic mice overexpressing TGFα under the control of metallothionein (MT) gene enhancer/promoter recapitulated the phenotypes of MD in the stomach4,5. Stomachs from MD patients also showed increased expression of TGFα, an EGF receptor (EGFR) ligand. Cetuximab, an anti-EGFR antibody, was reported to be the first effective medical treatment for MD, which confirmed that EGFR activation by TGFα overexpression contributes to the pathogenesis of MD6,7.
Since then, the MT-TGFα mouse line has been used as a mouse model for MD. Because TGFα expression is regulated by the heavy metal-response gene, metallothionein enhancer/promoter, zinc sulfate (ZnSO4) in drinking water or intraperitoneal injections of cadmium sulfate (CdSO4) have been used to induce TGFα expression5,8. The MT-TGFα mouse model has been further characterized for the phenotypes of MD. Using this mouse model, it has been shown that TGFα induces mucin-secreting surface foveolar cell differentiation while it inhibits differentiation into acid-secreting parietal cell and pepsinogen-secreting chief cell lineages9,10. It has also been shown that gastric body/fundic mucosa becomes antralized and that trefoil factor 2 (TFF2)-positive cells are present at the base of gastric glands, suggesting spasmolytic polypeptide-expressing metaplasia (SPEM) occurs in MD8,11.
In the current study, we show MT-TGFα mice develop features of MD without heavy metal treatment. These phenotypes include loss of Mist1 expression, a transcription factor that is essential for chief cell differentiation and is lost in SPEM. Due to the loss of Mist1, the Mist1-CreERT212 mouse line could not be utilized along with the MT-TGFα mouse line to examine if SPEM in MD arises from chief cells by lineage tracing. With the goal of overcoming the leakiness of the MT-TGFα mouse model, we developed a new MD mouse model (CMV-rtTA13; TetO-TGFα14) where TGFα is induced by doxycycline treatment (Doxi-TGFα). We confirmed this mouse model also displays features of MD. Using the Doxi-TGFα mouse model, we show that SPEM arises from chief cells by lineage tracing using the Mist1-CreERT2 mouse line. We introduce two MD mouse models in the current study. Both models can be used to investigate pathogenesis and to search for potential therapeutic targets. The new Doxi-TGFα mouse will be especially valuable when initiation of MD phenotypes needs to be precisely controlled or when genetic modification in chief cells is required using the Mist1-CreERT2 mouse line.
Protocol
All animal husbandry and procedures were approved by the Institutional Animal Care and Use Committees at Yale University and in accordance with U.S. Government Principles for the Utilization and Care of Animals Used in Research, Teaching, and Testing.
1. Mouse experiments
- Mouse treatments
- Treat MT-TGFα mice (both male and female at ages 2-4 months) with 25 mM ZnSO4 in drinking water for 2 weeks to induce TGFα overexpression.
- Treat CMV-rtTA/+; TetO-TGFα/+ (Doxi-TGFα) mice (both male and female at ages 2-4 months) with 2 mg/mL doxycycline in drinking water for 2 weeks to induce TGFα overexpression.
- Add one Mist1-CreERT2/+; R26-LSL-mTmG/+ male or female mouse and add one Doxi-TGFα mouse of the opposite sex in the breeding cage for the lineage-tracing of chief cells in MD mouse models.
- Inject Mist1-CreERT2/+; R26-LSL-mTmG/+; CMV-rtTA/+; TetO-TGFα/+ mice (both male and female at ages 2-4 months) with tamoxifen (37.5 mg/kg) intraperitoneally. Inject for 7 consecutive days, alternating between the right and left lower quadrants of the abdomen and avoiding hitting internal organs. Induce TGFα expression by doxycycline treatment (2 mg/mL) in drinking water for 2 weeks.
NOTE:Check for no tamoxifen-induced injury in the stomach15,16, as done here by counting the parietal cells in wild-type mice with and without tamoxifen treatment (Supplementary Figure 1).
- Stomach tissue fixation
- Euthanize mice by isoflurane overdose after 2 weeks of TGFα overexpression. Using a desiccator jar in a chemical fume hood, place mice on a perforated platform that prevents direct contact with the liquid anesthetic. Close the lid and monitor the mice until they lack respiration for more than 60 s, followed by cervical dislocation.
- Dissect out the stomach by cutting at the gastroesophageal junction, cutting the duodenum with 5 mm duodenum attached to the stomach, and removing all the peritoneal ligaments around the stomach using scissors and forceps (Figure 1A).
- Instill phosphate-buffered saline (PBS) using a 10 mL syringe with a pipette tip attached to the duodenal side. Squeeze it using fingers to remove luminal content. Repeat 3x (Figure 1B).
- Inflate the stomach with 2 mL of 10% formalin using another 10 mL syringe with a pipette tip attached. Clamp tightly with forceps at the gastroduodenal junction for 5-10 s while removing the tip from the stomach (Figure 1C).
- Submerge the inflated stomach in 10% formalin overnight at 4 °C with shaking (Figure 1D).
CAUTION: Use proper protective equipment when working with toxic substances such as formalin.
- Preparation of stomach for paraffin embedding
- Cut out the duodenum and the forestomach using a razor blade (Figure 1E). Rinse the stomach tissue with PBS for 5 min, 3x with shaking.
- Cut the stomach into 3-4 rings with the same thickness (3-5 mm) using a razor blade. Embed the stomach rings in 2% agarose to maintain orientation. Place the stomach rings sequentially from the proximal to the distal ones by positioning the lesser curvature side in the same direction (Figure 1F).
- Place the stomach rings embedded in agaroses in a tissue cassette (Figure 1G) and submit the samples to the histology core facility for paraffin embedding17.
2. Immunofluorescent staining
- Slide preparation
- Heat slides with paraffin sections on them in a dry oven or on a heat block set to 60 °C for a minimum of 1 h to overnight. Let cool to room temperature and place in a slide rack.
- Deparaffinization, rehydration, and antigen retrieval
- Label two slide jars, A and B, and fill each with enough Trilogy solution to fully submerge tissue.
- Place slide rack in Jar A and put both jars into a pressure cooker. Set the pressure cooker to high pressure for 15 min. Allow the pressure cooker to cool down before opening and retrieving slides.
- Immediately place the slide rack from Jar A into Jar B for 2 min to ensure the paraffin is removed from the slides. Remove slides from Jar B and place them into deionized (DI) water.
NOTE: Jar A can be filled with once-used Trilogy. New Trilogy should always be used in Jar B. - Place slides in Tris-buffered saline (TBS) for 5 min before proceeding to blocking.
- Blocking nonspecific background staining
- Remove slides from the slide rack and tap them sideways to remove excess TBS.
- Draw a box around the tissue using a hydrophobic marker. Apply blocking buffer to fully cover the tissue and incubate for 30-60 min in a humidified chamber at room temperature.
- Incubation with primary antibodies
- Select primary antibodies of target proteins raised in different host species and dilute in antibody diluent according to the manufacturer's recommendation. For this study, the following antibodies were used: H+/K+-ATPase, GIF, Mist118,19, and CD44v920,21. Detailed information on the primary antibodies can be found in the Table of Materials.
- Remove the blocking buffer, apply diluted antibodies, and place slides back in the humidified chamber. Incubate overnight at 4 °C.
- Remove primary antibodies and wash slides 3x in TBS for 5 min for each wash.
- Secondary antibodies and lectins
- Choose secondary antibodies according to the host species of the primary antibodies. Lectins can be mixed with secondary antibodies and applied at the same time.
- Dilute the secondary antibodies and lectins according to the manufacturer's recommendations in antibody diluent. Detailed information of secondary antibodies and lectins used in this study can be found in the Table of Materials.
- Apply secondary antibodies, lectins, and DAPI (1 µg/mL) and place slides back in the humidified chamber. Incubate for 1 h at room temperature away from light.
- Remove slides from the humidified chamber and rinse 3x with TBS for 5 min for each wash.
- Mounting and microscopy preparation
- Remove a slide from the TBS and remove excess fluid by tapping the slide sideways on a paper towel.
- Add 15-20 µL of mounting medium. Hold a clean coverslip by the edges and place one edge opposite the tissue with the mounting medium between.
- Slowly lower the coverslip so the medium is spread evenly over the tissue, being careful to avoid trapping bubbles.
- Use a task wipe to soak up excessive mounting medium around the edges. Dry the finished slide in the dark at room temperature before proceeding to microscopy. Repeat this process for the remaining slides.
Results
Adult wild-type (WT) and MT-TGFα mice received zinc sulfate (25 mM ZnSO4) in the drinking water for 2 weeks before sacrifice. The stomachs of WT mice appeared normal, grossly and histologically. In marked contrast, the stomachs of MT-TGFα were grossly thickened (Figure 2A). Microscopically, these stomachs showed massive foveolar hyperplasia with loss of both parietal and chief cells (Figure 2B-D), recapitulating the histological features of Ménétrier's disease (MD). Loss of chief cells can occur in two different ways: death of chief cells or spasmolytic polypeptide-expressing metaplasia (SPEM) arising from chief cells. To distinguish these two possibilities, we performed lineage tracing of chief cells by intercrossing Mist1-CreERT2; R26-LSL-mTmG mice with MT-TGFα mice. These mice were treated first with low dose tamoxifen (37.5 mg/kg intraperitoneally for 7 consecutive days) to label chief cells with GFP and then with ZnSO4 in the drinking water to induce TGFα overexpression. We observed rare GFP-labeled cells in the MT-TGFα mice (data not shown). In addition, there were no gross and histological differences in the MT-TGFα mice in the presence or absence of ZnSO4 (Figure 3A-C). One possible explanation for this finding was that TGFα may have reduced the expression of Mist1, an essential transcription factor for chief cells18, and subverting the usefulness of the Mist1-CreERT2 allele. In fact, immunohistochemical staining for Mist1 was lost in MT-TGFα mice without ZnSO4 treatment (Figure 3D).
To overcome the leakiness of the MT-TGFα mouse model, we turned to a doxycycline-inducible Doxi-TGFa transgenic model. This was achieved by intercrossing TetO-TGFα mice14 to a CMV-rtTA mouse line13; it has been reported previously that rtTA is expressed in the stomach. We confirmed that 2 weeks of doxycycline treatment induces foveolar hyperplasia and decreased numbers of parietal and chief cells in this new MD mouse model (Figure 4A-C). Compared to histomorphologic features in MT-TGFα mice, Doxi-TGFα mice showed less severe mucosal thickness and foveolar hyperplasia, as well as less of a decrease in parietal cells (Supplementary Figure 2). Nevertheless, we were able to lineage trace chief cells using the Mist1-CreERT2 mouse line and confirmed that chief cell lineages are also labeled with GSII and CD44v920,21, confirming that TGFα overexpression induces SPEM from chief cells (Figure 4D).
The current study shows that the MT-TGFα mouse model phenocopies the histopathological features of MD in the absence of heavy metal treatment, likely due to the intrinsic leakiness of the promoter/enhancer and/or the inability to avoid heavy metal exposure (Figure 3 and Supplementary Figure 2). Because TGFα suppresses Mist1 expression, the Mist1-CreERT2 mouse line cannot be used in the MT-TGFα mouse model. We generated a new MD mouse model in which TGFα expression can be induced by doxycycline treatment. Using this new Doxi-TGFα MD mouse model, we were able to confirm that TGFα overexpression induces SPEM derived from chief cells. The Doxi-TGFα MD mouse model will be useful when precise control of TGFα expression is necessary and also when genetic alteration in chief cells is required using the Mist1-CreERT2 mouse line.
Data availability:
All raw data are available as supplementary files.
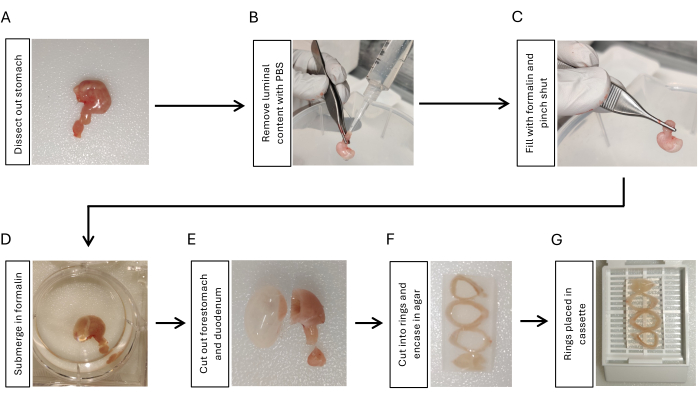
Figure 1: Workflow for fixation and preparation of gastric tissue embedding. (A) Dissect the stomach along with a short segment (3-5 mm) of the duodenum. (B) Remove luminal content by instilling PBS from the duodenum using a syringe with a pipette tip and squeezing it with fingers 3x. (C) Inflate the stomach with formalin from the duodenum using a syringe and pinch the gastroduodenal junction to retain the formalin. (D) Submerge the stomach in formalin and fix overnight at 4 °C with shaking. (E) Remove the forestomach and the duodenum with a razor blade and rinse with PBS. (F) Cut the gastric body and antrum into 3-4 rings in a cross-sectional manner and embed them in 2% agarose to maintain the orientation of stomach tissue rings. (G) Place the agarose-embedded tissue in cassettes. Please click here to view a larger version of this figure.
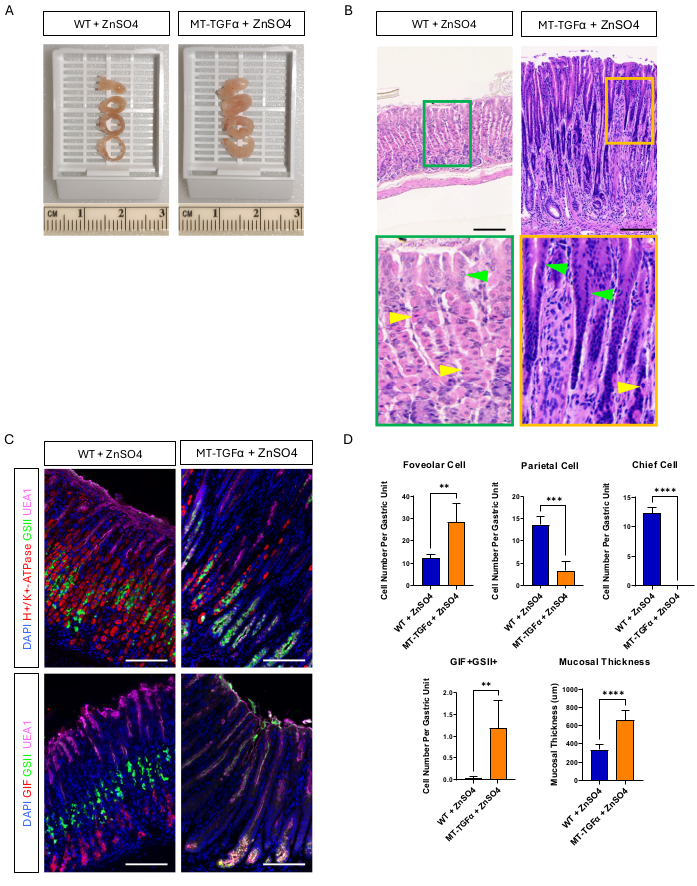
Figure 2: Gross and microscopic phenotypes of MT-TGFα mice treated with heavy metal ZnSO4. (A) The gastric wall is thicker in the MT-TGFα mice (right) compared to the control mice (left). (B) Stomach from MT-TGFα mice (right) shows massive foveolar hyperplasia and loss of parietal cells. Yellow arrowheads indicate parietal cells, and green arrowheads indicate foveolar cells. Scale bars represent 100 µm. (C) Immunofluorescent staining confirms that the MT-TGFα mouse stomach (right) shows increased numbers of foveolar cells (UEA1 positive) and SPEM cells (GIF and GSII double positive) and decreased numbers of parietal cells (H+/K+-ATPase positive) and chief cells (GIF single positive) compared to control mouse stomach (left). Scale bars represent 100 µm. (D) Quantification of different epithelial cell types and mucosal thickness in wild-type (WT) mice treated with ZnSO4 (n = 5) and MT-TGFα mice with ZnSO4 (n = 3). Error bars indicate standard deviation (**p < 0.01, ***p < 0.001, ****p < 0.0001). Please click here to view a larger version of this figure.
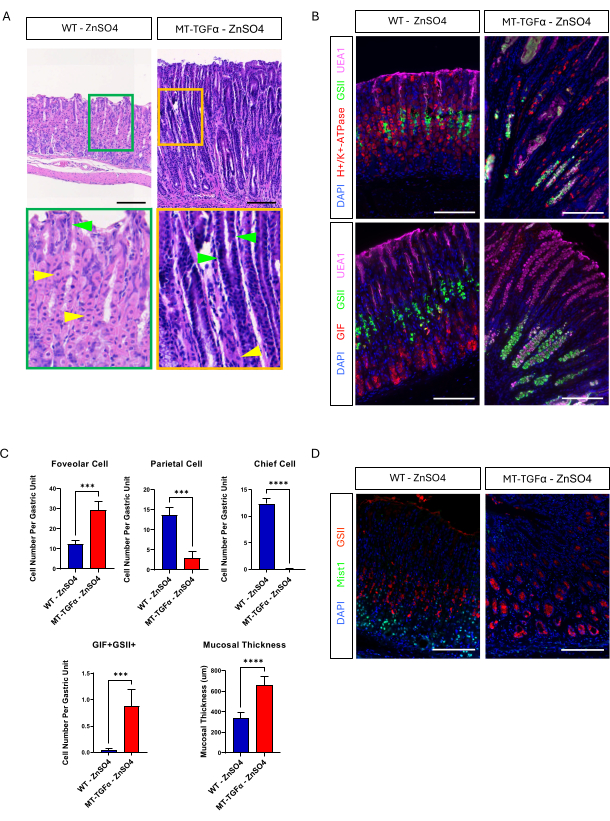
Figure 3: MT-TGFα mice develop gastric phenotypes without heavy metal treatment. (A) Stomach from MT-TGFα mice (right) shows massive foveolar hyperplasia and loss of parietal cells without heavy metal treatment. Yellow arrowheads indicate parietal cells, and green arrowheads indicate foveolar cells. Scale bars represent 100 µm. (B) Immunofluorescent staining confirms MT-TGFα mouse stomach (right) without heavy metal treatment shows increased numbers of foveolar cells (UEA1 positive) and SPEM cells (GIF and GSII double positive), and decreased numbers of parietal cells (H+/K+-ATPase positive) and chief cells (GIF single positive) compared to control mouse stomach (left). Scale bars represent 100 µm. (C) Quantification of different epithelial cell types and mucosal thickness in WT mice without ZnSO4 (n = 5) and MT-TGFα mice ZnSO4 (n = 3). Error bars indicate standard deviation (***p < 0.001, ****p < 0.0001). (D) Stomach from MT-TGFα mice (right) shows loss of Mist1 expression, which is normally expressed in the chief cells (left). GSII is a marker for mucous neck cells. Scale bars represent 100 µm. Please click here to view a larger version of this figure.
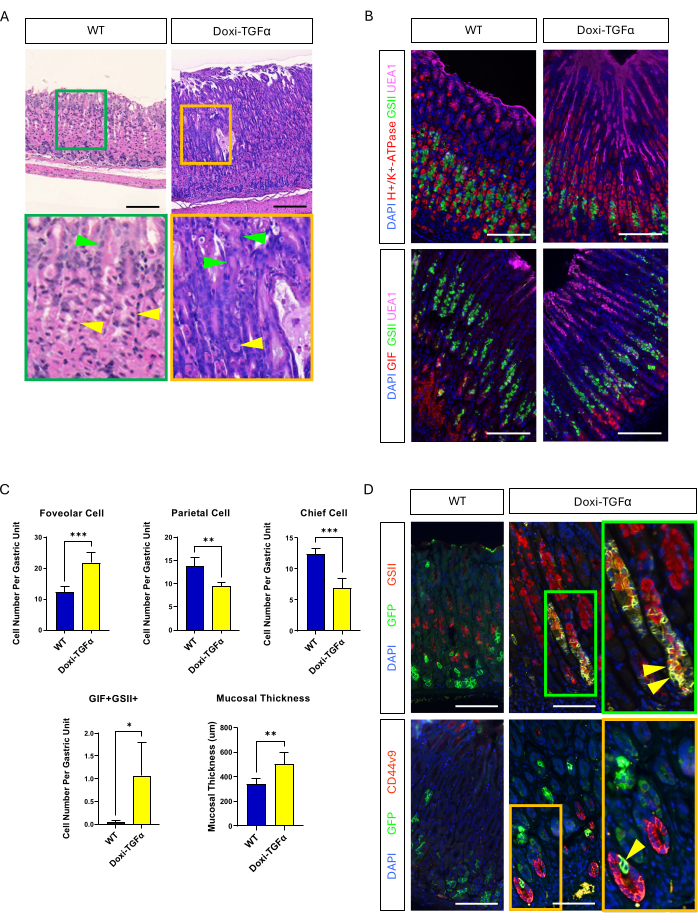
Figure 4: Doxycycline inducible TGFα mouse model (Doxi-TGFα) recapitulates Ménétrier's disease phenotypes, and SPEM formation is confirmed in this mouse model. (A) Stomach from CMV-rtTA; TetO-TGFα mice (Doxi-TGFα; right) shows massive foveolar hyperplasia and decreased number of parietal cells. Yellow arrowheads indicate parietal cells, and green arrowheads indicate foveolar cells. Scale bars represent 100 µm. (B) Immunofluorescent staining confirms Doxi-TGFα mouse stomach (right) shows increased numbers of foveolar cells (UEA1 positive) and SPEM cells (GIF and GSII double positive), and decreased numbers of parietal cells (H+/K+-ATPase positive) and chief cells (GIF single positive) compared to control mouse stomach (left). Scale bars represent 100 µm. (C) Quantification of different epithelial cell types and mucosal thickness in WT mice (n = 5) and Doxi-TGFα mice (n = 5). Error bars indicate standard deviation (*p < 0.05, **p < 0.01, ***p < 0.001). (D) Lineage tracing of chief cells (GFP positive) using Mist1-CreERT2; R26-LSL-mTmG mouse line reveals GFP positive cells are also positive for GSII and CD44v9 in the Doxi-TGFα mouse stomach, confirming SPEM formation derived from chief cells whereas control stomach (left) shows almost no GFP positive cells are also positive for GSII or CD44v9. Scale bars represent 100 µm. Please click here to view a larger version of this figure.
Supplementary Figure 1: Low dose tamoxifen (TMX) treatment does not induce gastric mucosal injury. Seven consecutive days of low-dose TMX treatment (37.5 mg/kg) does not induce gastric mucosal injury, exemplified by the maintenance of parietal cell number. (A) Representative H&E images without TMX treatment (left panel) and with TMX treatment (right panel). Scale bars represent 100 µm. (B) Quantification of parietal cell number per gastric unit in WT mice (n = 5) and WT with TMX treatment (n = 5). Please click here to download this File.
Supplementary Figure 2: Comparison of phenotypes among different Ménétrier's disease (MD) mouse models. (A) MT-TGFα with ZnSO4 treatment, MT-TGFα without ZnSO4 treatment, and Doxi-TGFα MD mouse models show foveolar hyperplasia, loss of parietal and chief cells, increased spasmolytic polypeptide-expressing metaplasia (SPEM) cells (GIF and GSII co-localized cells), and thickened mucosa. There are no phenotypic differences between MT-TGFα with and without ZnSO4 treatment groups. Doxi-TGFα mouse model shows less severe phenotypes than MT-TGFα mouse model. Please click here to download this File.
Discussion
Ménétrier's disease (MD) is a rare premalignant gastric disorder caused by TGFα overexpression1,2,3. Transgenic mice overexpressing TGFα under the control of the metallothionein gene enhancer/promoter (MT-TGFα) have been the only mouse model for MD to date4,5. Because the Metallothionein gene is heavy-metal responsive, zinc sulfate is added to drinking water, or cadmium sulfate is injected to induce TGFα in this mouse model5,8. One of the characteristic phenotypes in MD is the replacement of chief cells in the base of gastric glands by mucinous cells that are positive for markers present both in mucous neck cells and antral mucin cells. This can occur by either the death of chief cells or the reprogramming of chief cells to spasmolytic polypeptide-expressing metaplasia (SPEM)8,11. This can be tested by lineage tracing of chief cells in an MD mouse model. We tried to lineage trace the chief cells using the Mist1-CreERT2; R26-LSL-mTmG mice in which Mist1 positive chief cells are labeled with membrane-targeted GFP. However, we observed only a limited number of GFP-labeled cells in the MT-TGFα mice. This suggests either most of the GFP labeled chief cells died due to TGFα overexpression or the efficiency of GFP labeling in the chief cells was too low. Because it has been reported that there was no increase in cell death by TGFα overexpression8, we examined the possibility of a loss of Mist1 expression by evaluating MT-TGFα mice without heavy metal treatment. We were surprised by the complete phenotypes in MT-TGFα mice without heavy metal treatment, including foveolar hyperplasia and loss of parietal and chief cells. We also confirmed that Mist1 expression is lost in MT-TGFα mice even without heavy metal treatment. It has been previously reported that TGFα expression was not stringently controlled in MT-TGFα mice due to an inability to eliminate heavy metal exposure; however, TGFα expression was significantly increased by heavy metal treatment8. Comprehensive phenotypic evaluation has not been previously done in MT-TGFα mice without heavy metal treatment. The current study shows MT-TGFα mice develop complete phenotypic manifestation without heavy metal treatment.
Because Mist1 expression was lost in the MT-TGFα mice even before heavy metal treatment, there remained a need for another mouse model in which TGFα expression is specifically controlled so phenotypes do not develop without induction of TGFα overexpression. We generated a novel MD mouse model in which TGFα expression is induced by doxycycline treatment (Doxi-TGFα). We confirmed this mouse model induces foveolar hyperplasia and loss of parietal and chief cells, which are the major phenotypes of MD. We were able to lineage trace chief cells using the Mist1-CreERT2; R26-LSL-mTmG mice in this mouse model and found some GFP-labeled cells were also labeled with GSII and CD44v9, confirming that SPEM occurs in this novel MD mouse model. It is the first report confirming SPEM develops in the stomach with TGFα overexpression. SPEM is a more common premalignant gastric condition, which may be the reason gastric cancer is increased in MD patients22,23.
One caveat of using the Mist1-CreERT2 mouse line is that it has been reported that Mist1 can also be expressed in the gastric stem cells24. We found that a low dose (37.5 mg/kg) of tamoxifen treatment induces Cre activation, more specifically in the chief cells, with minimal activation in the isthmus region even at earlier time points before doxycycline treatment. Similar findings have been previously reported25. Lineage tracing using the Mist1-CreERT2; R26-LSL-mTmG mice shows GFP positive cells near GFP and SPEM marker (GSII and CD44v9) double-positive cells are mainly localized in the base where chief cells are located (Figure 4C). If the GFP and SPEM marker double-positive cells are traced from the stem cells, there should have been more GFP-positive cells toward the isthmus where stem cells are located. Alternatively, doxycycline-inducible GIF-rtTA mouse line26 or GPR30-rtTA mouse line27 could be used along with MT-TGFα mice for genetic modifications or lineage tracing of the chief cells. However, GIF-positive cells are decreased in MT-TGFα mice without ZnSO4 treatment (Figure 3B,C), and GPR30-positive cells are lost in SPEM, which occurs in MT-TGFα mice without ZnSO4 treatment. Therefore, they will be less efficient compared to Doxi-TGFα mice crossed with the Mist1-CreERT2 mouse line.
The current study shows that MT-TGFα mice develop complete phenotypic changes in the stomach without heavy metal treatment. We also show that one of the phenotypes is loss of Mist1 expression and this precludes the use of the Mist1-CreERT2; R26-LSL-mTmG mouse line to lineage trace chief cells. We generated a novel MD mouse model, Doxi-TGFα, and confirmed for the first time that SPEM develops in MD using this mouse model. This novel MD mouse model will be useful when precise control of the development of MD phenotypes is necessary and when genetic modifications in chief cells are needed using the Mist1-CreERT2 mouse line. MD phenotypes are induced by EGFR signaling activation by TGFα overexpression. However, it is not known EGFR activation in which cell types are responsible for the observed phenotypes. Using the Doxi-TGFα mouse model, we are currently investigating the role of EGFR signaling in different cell types, including chief cells utilizing the Mist1-CreERT2 mouse line.
Disclosures
All authors have no conflicts of interest to disclose.
Acknowledgements
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K08 DK124686 to WJH). We thank Gina Della Porta and Sarah E. Glass for editing the manuscript.
Author contribution:
TTG carried out experiments, generated figures, wrote the manuscript, and edited the manuscript. JDP carried out experiments, generated figures, wrote the manuscript, and edited the manuscript. SKM provided the TetO-TGFα mouse line and edited the manuscript. RJC conceived experiments and edited the manuscript. WJH conceived experiments, carried out experiments, interpreted data, searched the literature, wrote the manuscript, and edited the manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| Mouse anti H+/K+-ATPase antibody | A gift from Dr. Eunyoung Choi | N/A | Dilution: 1:10000 |
| Antibody Diluent | Abcam | 559148 | |
| Anti-rabbit GFP | Life Technologies Corporation | A11122 | Dilution: 1:500 |
| Charged Glass Slides | Fisher | 22-178-277 | |
| Cover Slips | Globe Scientific | 1411-10 | |
| Donkey anti-mouse 594 | Invitrogen | A12102 | Dilution: 1:500 |
| Donkey anti-rabbit 488 | Invitrogen | A21206 | Dilution: 1:500 |
| Donkey anti-rabbit 594 | Invitrogen | A21207 | Dilution: 1:500 |
| Donkey anti-rat 594 | Invitrogen | A21209 | Dilution: 1:500 |
| Doxyclycine Hyclate | TCI | D4116 | |
| Flouromount-G | Thermo fischer | 00-4958-02 | |
| GSII 488 | Invitrogen | L21415 | Dilution: 1:500 |
| Hydrophobic Marker | Electron Microscopy Sciences | 71312 | |
| Incubator | Labnet | I 5110 | |
| MIST1/bHLHa15 (D7N4B) XP Rabbit mAb | Cell Signaling | 14896S | Dilution: 1:200 |
| Pressure Cooker (6QT) | Cuisinart | CPC-600N1 | |
| Protein Block | Abcam | AB64226 | |
| Rabbit anti-GIF antibody | MyBioSource | MBS2028736 | Dilution: 1:200 |
| Rat anti CD44v9 mAb | Cosmo Bio | LKG-M002 | Dilution: 1:20000 |
| Slide Jars | Simport | M906-12AS | |
| Tamoxifen | Sigma-Aldrich | T5648 | |
| Trilogy | Sigma | 922P-10-RUO | |
| UEA1 Dylight 649 | Vector Laboratories | DL-1068 | Dilution: 1:500 |
References
- Huh, W. J., Coffey, R. J., Washington, M. K. Menetrier's disease: Its mimickers and pathogenesis. J Pathol Transl Med. 50 (1), 10-16 (2016).
- Tanksley, J. Jr, Tanksley, J. Pierre menetrier and his disease. Trans Am Clin Climatol Assoc. 123, discussion 133-124 126-133 (2012).
- Rich, A., et al. Distinguishing menetrier's disease from its mimics. Gut. 59 (12), 1617-1624 (2010).
- Takagi, H., Jhappan, C., Sharp, R., Merlino, G. Hypertrophic gastropathy resembling menetrier's disease in transgenic mice overexpressing transforming growth factor alpha in the stomach. J Clin Invest. 90 (3), 1161-1167 (1992).
- Dempsey, P. J., et al. Possible role of transforming growth factor alpha in the pathogenesis of menetrier's disease: Supportive evidence form humans and transgenic mice. Gastroenterology. 103 (6), 1950-1963 (1992).
- Fiske, W. H., et al. Efficacy of cetuximab in the treatment of menetrier's disease. Sci Transl Med. 1 (8), 8ra18(2009).
- Burdick, J. S., et al. Treatment of menetrier's disease with a monoclonal antibody against the epidermal growth factor receptor. N Engl J Med. 343 (23), 1697-1701 (2000).
- Goldenring, J. R., et al. Overexpression of transforming growth factor-alpha alters differentiation of gastric cell lineages. Dig Dis Sci. 41 (4), 773-784 (1996).
- Sharp, R., et al. Transforming growth factor alpha disrupts the normal program of cellular differentiation in the gastric mucosa of transgenic mice. Development. 121 (1), 149-161 (1995).
- Bockman, D. E., Sharp, R., Merlino, G. Regulation of terminal differentiation of zymogenic cells by transforming growth factor alpha in transgenic mice. Gastroenterology. 108 (2), 447-454 (1995).
- Nomura, S., et al. Evidence for repatterning of the gastric fundic epithelium associated with menetrier's disease and tgfalpha overexpression. Gastroenterology. 128 (5), 1292-1305 (2005).
- Shi, G., et al. Loss of the acinar-restricted transcription factor mist1 accelerates kras-induced pancreatic intraepithelial neoplasia. Gastroenterology. 136 (4), 1368-1378 (2009).
- Kistner, A., et al. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci U S A. 93 (20), 10933-10938 (1996).
- Hardie, W. D., et al. Conditional expression of transforming growth factor-alpha in adult mouse lung causes pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 286 (4), L741-L749 (2004).
- Keeley, T. M., Horita, N., Samuelson, L. C. Tamoxifen-induced gastric injury: Effects of dose and method of administration. Cell Mol Gastroenterol Hepatol. 8 (3), 365-367 (2019).
- Huh, W. J., et al. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology. 142 (1), 21-24.e7 (2012).
- Canene-Adams, K. Preparation of formalin-fixed paraffin-embedded tissue for immunohistochemistry. Methods Enzymol. 533, 225-233 (2013).
- Ramsey, V. G., et al. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires mist1. Development. 134 (1), 211-222 (2007).
- Huh, W. J., et al. Xbp1 controls maturation of gastric zymogenic cells by induction of mist1 and expansion of the rough endoplasmic reticulum. Gastroenterology. 139 (6), 2038-2049 (2010).
- Engevik, A. C., et al. The development of spasmolytic polypeptide/tff2-expressing metaplasia (spem) during gastric repair is absent in the aged stomach. Cell Mol Gastroenterol Hepatol. 2 (5), 605-624 (2016).
- Wada, T., et al. Functional role of cd44v-xct system in the development of spasmolytic polypeptide-expressing metaplasia. Cancer Sci. 104 (10), 1323-1329 (2013).
- Halldorsdottir, A. M., et al. Spasmolytic polypeptide-expressing metaplasia (spem) associated with gastric cancer in iceland. Dig Dis Sci. 48 (3), 431-441 (2003).
- Yamaguchi, H., Goldenring, J. R., Kaminishi, M., Lee, J. R. Identification of spasmolytic polypeptide expressing metaplasia (spem) in remnant gastric cancer and surveillance postgastrectomy biopsies. Dig Dis Sci. 47 (3), 573-578 (2002).
- Hayakawa, Y., et al. Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell. 28 (6), 800-814 (2015).
- Saenz, J. B., Vargas, N., Cho, C. J., Mills, J. C. Regulation of the double-stranded rna response through adar1 licenses metaplastic reprogramming in gastric epithelium. JCI Insight. 7 (3), e153511(2022).
- Caldwell, B., Meyer, A. R., Weis, J. A., Engevik, A. C., Choi, E. Chief cell plasticity is the origin of metaplasia following acute injury in the stomach mucosa. Gut. 71 (6), 1068-1077 (2022).
- Hata, M., et al. Gpr30-expressing gastric chief cells do not dedifferentiate but are eliminated via pdk-dependent cell competition during development of metaplasia. Gastroenterology. 158 (6), 1650-1666.e15 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved