Method Article
Minimally Invasive Anatomic Resection of Liver Segment VIII Based on Portal Territory to Treat Hepatocellular Carcinoma
* These authors contributed equally
In This Article
Summary
This study details laparoscopic S8 segmentectomy techniques, emphasizing the transition from partial to anatomical resection guided by portal pedicle navigation. Enhanced 3D anatomical comprehension, refined minimally invasive skills, and intraoperative ultrasound mastery improved procedural precision and safety, reducing complications while optimizing hepatic surgical outcomes through systematized anatomical resection protocols.
Abstract
Hepatectomy is the primary treatment for hepatocellular carcinoma (HCC) and is categorized into anatomical hepatectomy and non-anatomical hepatectomy based on the extent of resection. Anatomical hepatectomy utilizes the portal territory (PT) liver segment or subsegment as the basic anatomical unit, systematically resecting the tumor-bearing PT and completely removing the Glisson system that supplies and demarcates this area to enhance oncological efficacy. Non-anatomical hepatectomy follows the principle of radical oncological resection, emphasizing the removal of liver tissue more than 1 cm away from the tumor margin. With the popularization of precision surgery concepts, minimally invasive anatomical hepatectomy based on PTs has been widely applied. However, the minimally invasive resection of segment S8 of the liver is still considered one of the most challenging liver resections. We successfully performed an anatomical resection of portal territory segment S8 of the liver using intraoperative ultrasound, fluorescent laparoscopy, and Lannaec membrane dissection techniques, achieving good short-term clinical outcomes.
Introduction
Hepatocellular carcinoma, commonly known as liver cancer, is one of the most common malignant tumors in China. In 2022, there were 367,700 new cases of liver cancer in China, making it the fourth highest in terms of incidence; the number of deaths reached 316,500, making it the second leading cause of cancer-related deaths1. Hepatectomy provides one of the best opportunities for long-term survival in patients with HCC2. Liver resection can be classified into anatomic liver resection (AR) and non-anatomic liver resection (NAR) based on the extent of resection. AR involves the complete resection of anatomically independent liver segments or combined segments, along with the hepatic parenchyma within the tumor-bearing portal vein branches, to achieve better oncological outcomes and avoid complications from residual ischemic or congested areas. The advantage of AR is reflected in the thoroughness of tumor excision and the complete preservation of the inflow and outflow hepatic ducts of the remaining liver3. On the other hand, NAR, also known as irregular liver resection, refers to the resection of liver tissue more than 1 cm away from the tumor margin based on oncological radical resection principles. This surgical method does not strictly adhere to the anatomical segmentation of the liver but is tailored according to the location and size of the tumor, aiming to preserve as much normal liver tissue as possible while ensuring a safe margin for tumor excision.
With the advancement of precision surgery concepts and a deeper understanding of the liver's anatomy, the theory and practice of anatomical liver resection based on the portal territory (PT-AR) have gained recognition and initial promotion in recent years4. PT-AR involves preoperative three-dimensional reconstruction and basin analysis to identify the tumor-bearing portal territory (PT) and plan the surgery accordingly. Intraoperatively, liver segments or subsegments within the basin are used as basic anatomical units, with indocyanine green (ICG) fluorescence staining navigation being the primary method, supplemented by exposing representative intersegmental hepatic veins (IHVs). The liver is then dissected along physiological fissures to achieve complete resection of the tumor-bearing portal basin while ensuring the integrity and functional preservation of the future liver remnant (FLR). Superimposed intraoperative ultrasound-guided puncture for portal positive staining or retrograde staining after ligation of the target hepatic pedicle is a fundamental technical requirement for achieving PT-AR.
Laparoscopic liver resection is acknowledged for its minimally invasive approach and superior recovery outcomes when compared to traditional open surgery. However, the complexity of resecting different liver segments varies. The location of segment VIII of the liver deep within the upper abdomen, near the hepatic veins and inferior vena cava, along with the challenge of directly accessing the Glissonean pedicle of segment VIII, makes laparoscopic anatomical liver resection particularly challenging for this segment5,6,7,8,9.
This study demonstrates the portal venous regional anatomical resection of liver segment S8 for hepatocellular carcinoma. Our aim is to detail the technique and key steps of this surgery, including the laparoscopic ultrasound-guided puncture technique and liver pedicle dissection technique based on the Lannaec membrane. By sharing this protocol, we hope to provide evidence supporting the feasibility and safety of laparoscopic portal venous regional anatomical liver resection in the treatment of S8 hepatocellular carcinoma, ultimately improving patient treatment outcomes.
Protocol
The study involving laparoscopic anatomic liver resection for Segment 8 (LALR-S8) has adhered to standard ethical practices. It received approval from the Ethics Committee of the Shenzhen People's Hospital (LL-KY-2020462). Additionally, informed written consent was obtained from each patient, ensuring that the research complies with medical ethics norms and requirements.
1. Patient selection
- Use the following inclusion criteria:
- Perform LALR-S8 on patients with benign or malignant liver tumors and ensure that they undergo standard cardiopulmonary evaluations, blood tests, and biochemical assessments; ensure that they have no contraindications for surgery or anesthesia.
- Perform preoperative imaging, including abdominal CT angiography, three-dimensional reconstruction of the liver and vasculature, and enhanced MRI, as well as calculations of residual and standard liver volumes.
- Use the following exclusion criteria: patients with liver function classified as Pugh-Child Class C; those who cannot tolerate general anesthesia; patients with intrahepatic or extrahepatic metastases; those who have undergone open surgery; and patients who have received segmental resections or other combined surgical treatments.
2. Preoperative preparation, surgical position, and anesthesia
- Preoperative preparation
- History and physical examination: Assess liver function, coagulation profile, ICG clearance test, and overall health status.
- Imaging: Obtain detailed preoperative imaging (e.g., enhanced abdominal CT, MRI, and three-dimensional reconstruction of the liver and vasculature) to delineate the anatomy of segment 8 of the liver and its vasculature (Figure 1)
- Fasting: Ensure the patient adheres to fasting instructions, typically starting from midnight the day before the surgery.
- Medications: Administer prophylactic antibiotics if necessary and review any medications that may impact bleeding or liver function.
- Informed consent and education: Explain the laparoscopic procedure, including its methods, risks, and benefits, and obtain informed consent.
- Surgical position
- Supine position: Position the patient on the operating table in the supine position.
- Reverse Trendelenburg position: Slightly tilt the operating table to facilitate exposure and access to the liver.
NOTE: This position helps to move the liver upwards and away from the upper abdomen. - Stabilization: Secure the patient to the operating table to prevent movement during the procedure.
- Anesthesia
- General anesthesia: Administer general anesthesia to ensure the patient remains unconscious and comfortable throughout the surgery.
- Induction and maintenance: Use intravenous induction agents (e.g., propofol) and muscle relaxants (e.g., succinylcholine) for intubation. Maintain anesthesia with inhaled agents (e.g., sevoflurane) and supplemental analgesics (e.g., fentanyl). Adjust the depth of anesthesia to ensure adequate anesthesia and patient safety.
- Monitoring: Continuously monitor heart rate, blood pressure, blood oxygen saturation, and end-tidal carbon dioxide levels.
3. Surgical techniques
- Following intravenous-inhalation anesthesia, position the patient in a 30° left lateral decubitus position with head elevation and legs separated. Use a five-port approach for liver resection, with an insufflation pressure maintained at 11-13 mmHg, central venous pressure at 3-5 cmH2O, and the Pringle maneuver applied for 10-15 min of occlusion followed by a 5 min release.
- Make a vertical incision 2 cm below the right edge of the umbilicus, and open the abdominal wall layers sequentially to access the abdomen. Insert a 12 mm trocar to establish pneumoperitoneum, then introduce the laparoscope into the abdominal cavity.
- Place five trocars as follows: one 12 mm trocar in the suprumbilical region for observation; one 5 mm trocar at the right anterior axillary line; one 12 mm trocar below the right medial clavicle; one 5 mm trocar horizontally 2 cm below the xiphoid process; and one 12 mm trocar 2 cm above the umbilicus (Figure 2).
NOTE: Common surgical instruments used in liver resection include intraoperative laparoscopic ultrasound, Harmonic scalpel, and bipolar coagulation devices. - Use intraoperative laparoscopic ultrasound (LUS) to guide the puncture, with ICG for positive staining of the anatomical resection of liver segment S8:
- Dissection of surrounding ligaments and identification of P8: Dissect the surrounding ligaments of the right liver lobe and use the LUS probe to identify the portal vein branch for segment 8 (P8), adjusting the puncture position and needle angle as necessary.
- Ultrasound probe insertion and positive staining with ICG: Insert the BK laparoscopic ultrasound probe through the 12 mm trocar port. Puncture the P8 with a 21 G percutaneous transhepatic cholangiography (PTC) needle guided by the LUS. Inject 5-10 mL of 1.25% ICG through the needle to stain segment VIII, ensuring no retrograde flow into adjacent segments (Figure 3)
- Negative staining of the anatomical resection of liver segment S8 via the hilar approach:
- Dissection of the S8 hepatic pedicle: Expose the right anterior liver hilum based on the Lannaec membrane between the gate VI and gate V10. Then, dissect along the ventral side of the right anterior hepatic pedicle and towards the cephalad side on the left to expose the S8 hepatic pedicle; mobilize and lift this pedicle.
- Hemostasis and negative staining: After hilum dissection, apply a vascular clamp for hemostasis of the S8 hepatic pedicle (Figure 4) and confirm the ischemic line of segment S8 to include the tumor. Inject 5-10 mL of 1.25% ICG through the peripheral vein. Look for segment VIII that will be delineated by the fluorescence after 5 min (Figure 5).
- Liver resection guided by fluorescent interface: Monitor fluorescence to confirm that the stained area covers the tumor. Make incisions along the interface between fluorescent and non-fluorescent areas using an ultrasonic scalpel or CUSA, while preserving intersegmental hepatic veins (Figure 6).
NOTE: Both the tumor and the PT hepatic segment or subsegment can be resected completely in this way. - Hemostasis and inspection: Achieve hemostasis by electrocautery or suturing after lesion resection. Inspect the resection site to confirm there is no residual bleeding or bile leakage. On the cross-section, observe the intersegmental veins and the P8 segment end (Figure 7).
Results
Between January 2022 and December 2023, a total of 17 patients underwent hepatectomy of segment S8. Of these, seven cases involved non-anatomical resections, six cases were anatomical resections via the hepatic parenchymal approach, and four cases were portal territory anatomical resections. There were no significant differences in preoperative Child-Pugh scores, tumor size, and liver reserve function among the groups. The surgical time for the PT anatomical resection group was longer than that for the non-anatomical resection and hepatic parenchymal approach anatomical resection groups, primarily due to the relatively challenging portal puncture or dissection of the corresponding liver segments, which required more time. There were no significant differences in intraoperative blood loss among the groups, but the peak ALT level postoperatively was lower in the portal territory anatomical resection group compared to the other two groups, likely because this group had less residual non-functional liver tissue. All surgical margins exceeded 1 cm, with the PT anatomical resection group having larger margins than the other two groups. No bile leakage occurred in any case, and there were no unplanned reoperations. One-way ANOVA analysis was employed for count data, and the rank-sum test was utilized for measurement data. Specific results are detailed in Table 1.
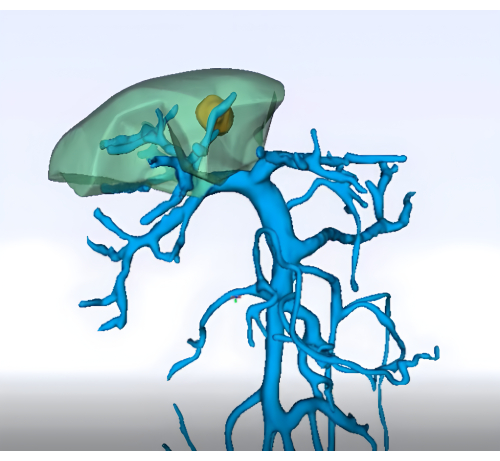
Figure 1: Preoperative analysis of the portal vein territory using three-dimensional reconstruction technology. Please click here to view a larger version of this figure.

Figure 2: Trocar placement of laparoscopic hepatic segment VIII resection. Please click here to view a larger version of this figure.
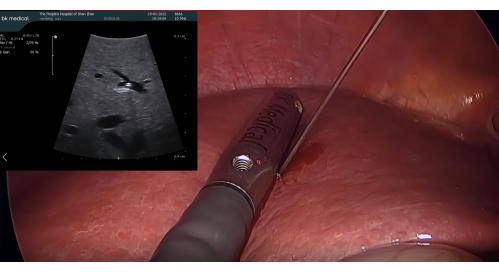
Figure 3: Puncture of P8 with a 21 G PTC needle guided by the laparoscopic ultrasound probe. Abbreviation: PTC = percutaneous transhepatic cholangiography. Please click here to view a larger version of this figure.
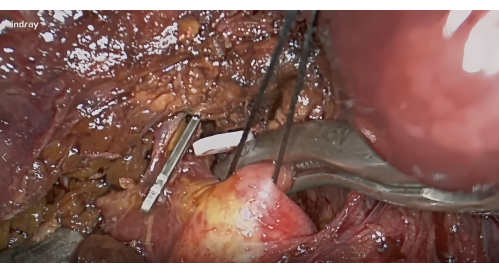
Figure 4: Dissection of the hepatic pedicles of segment VIII along the Lannaec membrane and blockade with vascular clamp. Please click here to view a larger version of this figure.

Figure 5: Marking the resection line after negative staining. Please click here to view a larger version of this figure.
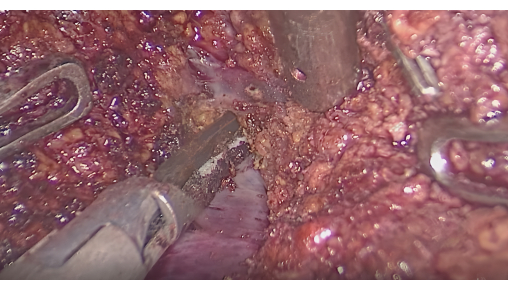
Figure 6: Dissection of the liver parenchyma between intersegmental veins. Please click here to view a larger version of this figure.

Figure 7: Intersegmental vein and the P8 stump in the resection section. Please click here to view a larger version of this figure.
| non-anatomical resection (n=7) | anatomical resections through a parenchymal approach (n=6) | portal venous territory-based anatomical resection (n=4) | F value/χ2 | p-value | |
| Preoperative Child-Pugh score | 5.4±0.8 | 5.2±0.4 | 5.3±0.5 | 0.306 | 0.741 |
| Tumor size (cm) | 3.4±1.5 | 3.1±0.8 | 2.8±0.6 | 0.454 | 0.644 |
| Preoperative ICG 15 min retention rate (%) | - | - | - | 0.195 | 0.907 |
| Operative time (min) | 189±52 | 201±36 | 268±51 | 3.826 | 0.047 |
| Blood loss (mL) | 228±135 | 175±52 | 170±24 | 0.716 | 0.506 |
| Minimum surgical margin (cm) | 1.3±0.5 | 1.7±0.3 | 2.0±0.4 | 3.972 | 0.043 |
| Peak postoperative ALT (U/L) | 328±109 | 219±45 | 152±38 | 7.045 | 0.008 |
| Postoperative day 5 bilirubin levels(μmol/L) | 17.2±8.2 | 18.1±4.2 | 14.7±4.3 | 0.389 | 0.685 |
| Postoperative discharge time (days) | 7.5±1.3 | 6.7±0.8 | 6.3±1.0 | 2.279 | 0.139 |
| Postoperative bile leakage | 0 | 0 | 0 | - | - |
| Unplanned reoperation | 0 | 0 | 0 | - | - |
Table 1: Comparison of clinical data between different groups.
Discussion
The resection of segment S8 of the liver, particularly anatomical resection, remains a significant challenge11. The theoretical basis of portal pedicle-based anatomic hepatectomy has yet to gain widespread acceptance within the hepatic surgical community. Consequently, hepatobiliary surgeons continue to pursue robust clinical evidence to objectively evaluate the therapeutic value of anatomic liver resection. Recent advancements in hepatic anatomical re-conceptualization, particularly regarding Laennec's capsule and the six-sector theory, have theoretically enabled precise identification and dissection of individual hepatic pedicles corresponding to specific segments or subsegments. The integration of fluorescent laparoscopy and laparoscopic ultrasonography has further facilitated the realization of laparoscopic anatomical hepatectomy, permitting real-time visualization of territorial perfusion boundaries and intraoperative navigation. According to the Tokyo expert consensus conference of 202112, anatomical resection is defined as the complete removal of liver portions associated with specific portal vein territories, delineated by tertiary portal vein branches. Anatomical liver resection is characterized by the complete excision of anatomically independent liver segments, subsegments, or combined segments11,13. Compared to non-anatomical liver resection, anatomical resection simultaneously removes the corresponding liver segments associated with the relevant portal vein branch territory, theoretically reducing the risk of tumor dissemination through portal venous blood flow in the affected liver segment and minimizing postoperative complications. Consequently, it is currently regarded as the preferred surgical approach for liver cancer14.
The intersegmental or segmental planes of the liver are determined by the boundaries of portal vein territories. The hepatic veins traversing these planes are referred to as intersegmental veins, which serve as important landmarks during liver resection, allowing for accurate identification through continuous exposure at the cutting plane15. This method necessitates expertise in liver ultrasound imaging, proficient use of LUS, and puncture techniques, which require a learning curve. Based on our experience, the skilled application of intraoperative ultrasound is crucial for enhancing surgical success rates and patient safety. Surgeons at all levels should start by familiarizing themselves with ultrasound imaging recognition and interpretation through routine percutaneous ultrasound interventions, mastering basic operational skills. Subsequently, they should progress to the application of ultrasound in open surgeries, learning how to utilize ultrasound for guiding liver resections and assessing liver tumors. This phase of training will bolster surgeons' understanding of intraoperative anatomical structures. Ultimately, practitioners should aim to master the use of intraoperative ultrasound in laparoscopic procedures to achieve greater precision, especially in situations with limited visualization. Through this gradual learning and practice process, surgeons will become adept at employing ultrasound technology in complex surgeries, significantly enhancing both safety and efficacy.
The LALR-S8 technique aims to effectively locate the vascular supply to segment S8 and define the intersegmental plane. Makuuchi et al. were the first to propose a method for targeting the portal vein under ultrasound guidance, followed by dye injection to clarify the perfusion of the tumor segment3. In 2008, Aoki et al. pioneered the application of fluorescence imaging technology for segment localization during liver surgery16. Currently, the ICG fluorescence-guided method under LUS has become a standard technique to enhance the accuracy of laparoscopic anatomical liver resections17. This approach not only addresses the limitation of ischemic lines being visible only on the liver surface but also provides real-time visualization of clear fluorescent demarcation lines on the intersegmental plane. The Glissonean approach with ICG fluorescence staining enables anatomical liver resection while maximizing the preservation of functional residual liver tissue and minimizing intraoperative bleeding.
Makuuchi proposed that anatomical liver resection for segment S8 should include four steps: (1) marking the liver segment boundaries on the surface using staining or blood flow occlusion techniques; (2) performing liver parenchyma resection guided by ultrasound, using the landmark vein of that liver segment as a boundary; (3) achieving full exposure of significant veins at the liver cut surface; (4) ligating the Glisson system near the root of the liver segment3. The portal vein branches for segment S8 mainly consist of the ventral and dorsal branches. The dorsal branch of segment S8 typically bifurcates near the root of the right anterior portal vein, while the portal vein branch of segment S5 bifurcates at its terminal side, necessitating separate puncture staining for the ventral and dorsal branches. There is a prominent branch of the middle hepatic vein (V8) running between the dorsal branch of segment S8 and the right anterior portal vein, responsible for venous drainage from S8. After total resection of segment S8, the middle hepatic vein, right hepatic vein, and inferior vena cava can be seen at the cut surface.
From January 2022 to December 2023, a total of 17 patients underwent liver S8 segmentectomy. All patients recovered and were discharged without any major complications. Our experience in liver surgery emphasizes the integration of theoretical knowledge and practical skills, particularly in detailed intrahepatic anatomy learning, recommending dissection practice using cadaveric livers. Additionally, we stress the importance of mastering laparoscopic techniques through simulation training and participation in minimally invasive procedures. Familiarity with various endoscopic instruments, such as harmonic scalpels and intraoperative ultrasound, is crucial. Furthermore, leveraging on-site training and surgical debriefings to understand the theory and techniques of anatomical liver resection is essential, with a focus on gradually increasing procedural complexity and ultimately achieving simplification. Proficient application of intraoperative ultrasound, close collaboration within the surgical team, and thorough self-review of surgical performance are equally critical. An effective intraoperative rescue mechanism is vital, ensuring timely conversion to open surgery when necessary and securing support from senior surgeons to enhance procedural success.
From our experience, we have the following insights: (1) Master 3D Segmental Anatomy via cadaveric training focusing on Glissonean pedicle microdissection. (2) Refine Laparoscopic Skills through simulation-based minimally invasive drills for instrument precision. (3) Optimize Energy Devices (ultrasonic scalpel/CUSA/ICG fluorescence) for safe parenchymal transection. (4) Implement Multimodal Learning, combining video analysis and case debriefings for AR strategy optimization. (5) Enhance Team Synergy with anesthesiologist-managed CVP control and proficient surgical assistance. (6) Establish Rescue Protocols defining open conversion thresholds and senior surgeon escalation pathways.
However, this study has some limitations, as it involves only a few cases. Additionally, the study is retrospective, and patient selection is limited. More precisely designed prospective studies are needed to further compare the advantages and disadvantages of different minimally invasive liver resection techniques. We will share our experiences promptly upon obtaining more reliable data.
Disclosures
The authors have no conflicts of interest or financial ties to disclose.
Acknowledgements
This work was supported by grants from the Project of Guangdong Provincial Basic and Applied Basic Research Fund (No. 2023A1515220114); the Science and Technology Major Project of Shenzhen Municipal Science and Technology Innovation Commission, (No.KJZD20230923114120038), Shenzhen Key Medical Discipline Construction Fund (No.SZXK015); and Guangdong Provincial and National Key Clinical Specialty Construction Project and National Key Clinical Specialty Construction Project.
Materials
| Name | Company | Catalog Number | Comments |
| Bipolar electric coagulation forceps | Mindray | Seal 7 | For blood vessel coagulation and division |
| Fluorescence Endoscopic camera system | Mindray | R1 | An endoscopic camera system with 4K fluorescence imaging |
| Intraoperative Ultrasonic imaging system | ALOKA | UST-5418 | With the four-directional flexible linear array ultrasonic laparoscopic transducer, the intraoperative ultrasound support ultrasonic elastography, contrast ultrasound, and magnetic navigation guided puncture |
| Intraoperative Ultrasonic imaging system | Mindray | LAP13-4Cs | Four-directional flexible linear array ultrasonic laparoscopic transducer which supports support ultrasonic elastography and contrast ultrasound |
| SPSS 20.0 | statistical analysis software | ||
| Ultrasonic scalpel | Johnson & Johnson | ETHICON GEN11 | For blood vessel coagulation and division |
References
- Zheng, R. S., et al. Cancer incidence and mortality in China, 2022. Chinese Journal of Oncology. 46 (3), 221-231 (2024).
- Qin, S. Primary liver cancer diagnosis and treatment Expert Panel of the Chinese Ministry of Health Guidelines on the diagnosis and treatment of primary liver cancer (2011 edition). Chin Clin Oncol. 1 (1), 10(2012).
- Makuuchi, M., Hasegawa, H., Yamazaki, S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet. 161 (4), 346-350 (1985).
- Wang, X., Cao, J., Li, J. Anatomic liver resection based on portal territory with margin priority for hepatocellular carcinoma. JAMA Surg. 159 (6), 710-711 (2024).
- Urade, T., et al. Laparoscopic anatomical liver resection using indocyanine green fluorescence imaging. Asian J Surg. 43 (1), 362-368 (2020).
- Fuks, D., Aldrighetti, L., Jiao, L. R., Wakabayashi, G., Limongelli, P. Laparoscopic management of hepatocellular carcinoma: A critical reappraisal. Surg Laparosc Endosc Percutan Tech. 27 (4), 203-205 (2017).
- Morimoto, M., et al. Minimally invasive anatomic liver resection: Results of a survey of world experts. J Hepatobiliary Pancreat Sci. 29 (1), 33-40 (2022).
- Goh, E. L., Chidambaram, S., Ma, S. Laparoscopic vs open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: A meta-analysis of the long-term survival outcomes. Int J Surg. 50, 35-42 (2018).
- Ban, D., et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci. 21 (10), 745-753 (2014).
- Sugioka, A., Kato, Y., Tanahashi, Y. Systematic extrahepatic Glissonean pedicle isolation for anatomical liver resection based on Laennec's capsule: proposal of a novel comprehensive surgical anatomy of the liver. J Hepatobiliary Pancreat Sci. 24 (1), 17-23 (2017).
- Xie, Q., Gao, F. A commentary on 'approaches of laparoscopic anatomical liver resection of segment 8 for hepatocellular carcinoma: a retrospective cohort study of short-term results at multiple centers in China'. Int J Surg. 111 (1), 1646-1647 (2023).
- Ciria, R., et al. Study group of Precision Anatomy for Minimally Invasive Hepato-Biliary-Pancreatic surgery (PAM-HBP surgery). A snapshot of the 2020 conception of anatomic liver resections and their applicability on minimally invasive liver surgery. A preparatory survey for the Expert Consensus Meeting on Precision Anatomy for Minimally Invasive HBP Surgery. J Hepatobiliary Pancreat Sci. 29 (1), 41-50 (2022).
- Morimoto, M., et al. and Study group of Precision Anatomy for Minimally Invasive Hepato-Biliary-Pancreatic surgery (PAM-HBP surgery). Glissonean approach for hepatic inflow control in minimally invasive anatomic liver resection: A systematic review. J Hepatobiliary Pancreat Sci. 29 (1), 51-65 (2022).
- Fuks, D., Aldrighetti, L., Jiao, L. R., Wakabayashi, G., Limongelli, P. Laparoscopic management of hepatocellular carcinoma: A critical reappraisal. Surg Laparosc Endosc Percutan Tech. 27 (4), 203-205 (2017).
- Monden, K., et al. Landmarks and techniques to perform minimally invasive liver surgery: A systematic review with a focus on hepatic outflow. J Hepatobiliary Pancreat Sci. 29 (1), 66-81 (2022).
- Kang, L. -M., Zhang, F. -W., Yu, F. -K., Xu, L. Pay attention to the application of indocyanine green fluorescence imaging technology in laparoscopic liver cancer resection. World J Clin Cases. 12 (23), 5288-5293 (2024).
- Cassinotti, E., et al. European Association for Endoscopic Surgery (EAES) consensus on Indocyanine Green (ICG) fluorescence-guided surgery. Surg Endosc. 37 (3), 1629-1648 (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved