Method Article
Administration of Volatile Anesthetics to Zebrafish Larvae for Behavioral Observation
In This Article
Summary
This protocol presents an accessible method for creating a gas-tight environment to administer volatile anesthetics to larval zebrafish for behavioral experiments.
Abstract
This study describes a unique method for administering volatile anesthetics, such as isoflurane and sevoflurane, to larval zebrafish during behavioral experiments. While zebrafish offer numerous advantages as a vertebrate model organism -- including complex behaviors, genetic tractability, transparent embryos, and rapid development -- their use in studying volatile anesthetics has been limited. The administration of volatile anesthetics often requires complex or cumbersome apparatuses that may not be broadly accessible, creating barriers to the pharmacologic study of volatile anesthetics in aqueous model organisms. This method presents a straightforward technique using adhesive silicone sheets to create a gas-tight seal on glass 96-well plates. Validation was performed through the assessment of spontaneous movement, which showed no significant differences between sealed and open wells over a 90-min period. Additionally, anesthetic concentration remained stable over time, as measured by HPLC. Representative results include the experimental determination of median effective concentration (EC50) values for sevoflurane. This study provides a simple and accessible approach for pharmacologic experiments using volatile anesthetics, which can be easily adapted to study other volatile agents and experimental endpoints.
Introduction
Non-aquatic animal models (worms, flies, rodents, rabbits, primates, etc.) have historically been the models most commonly used to study anesthetic pharmacology. In each of these species, volatile anesthetics (administered through inhalation of gas) are far less challenging than the administration of intravenous anesthetics (administered by either intravenous or intraperitoneal routes)1. In addition to the technical challenge of simply administering the intravenous drugs, achieving a steady state of anesthesia is far easier with volatile agents than intravenous agents2.
Administration of intravenous anesthetics to larval aquatic species can be achieved by diffusion from their surrounding aqueous medium3,4, and is thus more akin to the administration of volatile anesthetics for non-aqueous species. For many years, tadpoles (Xenopus laevis) were the model of choice for studying anesthetics due to the ease of drug administration and steady-state pharmacokinetics achieved by simple bath addition of the anesthetic to their artificial pond water5,6. However, there are relatively few examples of the study of volatile anesthetics in this model due to the requirement of gas-tight apparatuses that are often purpose-built and can be cumbersome to use7,8.
In recent years, the zebrafish has become a preferred model for the study of intravenous anesthetics due to the same ease of drug administration in addition to other advantages such as their well-described complex behaviors and availability of commercial units to observe these behaviors9. Many recent studies have employed such commercially available observation units to screen for neuroactive compounds, including anesthetics, using 96-well and 24-well plate formats to quantify the behavior of the zebrafish larvae4,10,11,12,13,14,15. The larval stages are particularly well-suited for pharmacology studies due to their ability to assess many behavioral responses, their small size, high throughput compatibility, and well-characterized neural circuits9,16,17. However, one significant limitation in these studies of anesthetics in the zebrafish model is the relative difficulty in administering volatile anesthetics. This limitation has led to scant examples of comparison between volatile and intravenous agents4,15,18. Being able to easily study and compare both classes of anesthetics in a single animal model adds an important tool to facilitate the study of anesthetic pharmacology across all classes.
Traditionally, the study of intravenous anesthetics in an aquatic species has required specialized, often custom, equipment that can be difficult to use or may not be sufficiently gas-tight to prevent loss of the anesthetic for the duration of the experiment. The procedure described here presents an easily accessible alternative means of producing a gas-tight seal in a glass chamber that is suitable for observation of larval zebrafish behavior using a commercial observation unit (see Table of Materials).
Protocol
All zebrafish experiments were conducted in accordance with animal use protocols approved by the Institutional Animal Care and Use Committee (IACUC). Adult zebrafish were maintained at the University of Pennsylvania's aquatic facility under the oversight of the University Laboratory Animal Resources (ULAR). Tübingen long-fin wild-type zebrafish were bred in-house for all experiments and maintained under standard husbandry conditions with a 13/11-h light/dark cycle until they reached 5 days post-fertilization (dpf). Experiments were conducted using biological replicates, with clutches derived from distinct mating pairs to ensure adequate biological diversity. Details of the reagents and equipment used in this study are listed in the Table of Materials.
1. Fabrication of adhesive silicone sheets
NOTE: Equivalents of this material (silicone sheet plus adhesive) can be commercially sourced, but often at greater expense and lesser availability.
- Lay out the 0.5 mm silicone sheet on a clean, flat surface.
- Inspect the sheets for any creases and avoid them when possible.
NOTE: Silicone sheets with creases may not adequately adhere to the tape and may result in inadequate sealing of the wells and/or poor visualization of the zebrafish. - Clean the sheets with 70% alcohol to remove any oil or debris and allow them to dry.
- Using an applicator, such as a paintbrush, apply a thin, even coat of adhesive primer to the silicone sheet. To avoid over-application, work in smaller sections as the adhesive promotor dries quickly (sets in 1-5 s).
NOTE: Without this adhesion promotor, the tape will not adequately adhere to the silicone sheet. - Once the adhesive promotor is applied, align the double-coated tape with the silicone sheet with the paper backing facing up.
- Gradually apply the tape to the silicone using a roller to smooth the surface and to remove air bubbles (after initial application, working silicone-side-up will aid in visualizing and removing air bubbles).
- Allow the adhesive promoter to fully cure for 24 h on a flat surface.
- Cut the adhesive silicone into strips at least 10 mm wide (7.5 mm well diameter plus overlap on each side).
NOTE: When using every other row of the well plate, wider variance in strips will not negatively impact wells in adjacent rows, which may not only make application easier but also be necessary to achieve a seal around the entire circumference of the well.- Ensure that strips are long enough to cover at least the width of one row of the well plate.
NOTE: If using a well plate that is not 96-well, such as a 24-well or 48-well plate, the strip sizes can be adjusted or cut to fit the specific dimensions of the plate, ensuring proper coverage regardless of plate size.
- Ensure that strips are long enough to cover at least the width of one row of the well plate.
2. Preparation of solutions
NOTE: All stock and assay solutions of volatile anesthetics were made right before the experiment to avoid loss of volatile compound concentration.
- For each solution (stock or working solution), add a volume of non-volatile solvent (E3 embryo water, DMSO, etc.) to an HPLC or scintillation vial that when combined with the final dilution of volatile anesthetic, the total volume will sufficiently fill the vial leaving minimal headspace (see Figure 1A).
- Add volatile anesthetic with a Hamilton gas-tight syringe and quickly seal the vial.
NOTE: Stock solutions in vials with septum lids can be used to facilitate further dilutions via the transfer of solution with gas-tight syringes. - Then, mix the vials (vortex, sonication) as needed and temporarily store them while preparing zebrafish plates.
3. Setup of the behavioral experiment
NOTE: This step of the protocol may take some practice. It is recommended to work without fish or solutions containing precious material until comfortable with the technique.
- Transfer one larval zebrafish (5 dpf) into every other row of the glass 96-well plate.
NOTE: Leaving every other row empty allows for reduced potential for contamination of adjacent wells from spillover as the wells are sealed in step 3.4. - Carefully remove the E3 solution from each well. This can be done one row at a time or on the entire plate if it is able to work quickly.
NOTE: The duration for which larvae were out of the solution was minimized to reduce potential stress. Alternative procedures may be used based on different experimental needs, including only partial removal of the solution before the addition of the drug-containing solution to minimize stress. The protocol should be adapted to suit the needs of the experiment, including equilibration time given to the animals to compensate for any potential stress, solution mixing efficiency, and accuracy of concentrations of a drug-containing solution, if not all of the E3 is removed prior to addition of the working solution. - Next, use a transfer pipette to fill each well in the row with the experimental solution. Take care not to introduce bubbles. Overfill each well (Figure 1B).
- Using the adhesive silicone strips made in step 1, press the strip downward quickly (Figure 1C). This helps prevent bubble formation and keeps fish from overflowing into adjacent wells.
- Press firmly on the top of the plate to ensure each well is sealed.
- Repeat steps 2-5 until the solution is exchanged and the wells for every other row of the well plate are sealed (Figure 1D).
- Gently turn the plate over and inspect for air bubbles.
NOTE: The presence of air bubbles will obscure visualization of zebrafish movement. - Place the plate into the behavior observation unit (silicone adhesive side down) and perform the behavioral experiment (see the Results section for details).
Results
Comparison to traditional open-well observation
In order to assess whether adhesive sealing has any effect on spontaneous movement, a comparison was made between the controls in open versus sealed wells. As seen in Figure 2A, no statistically significant difference in movement was observed between these groups for the standard ~30 min duration used in the experiments described in this article. Recognizing that other experimental paradigms might need longer experimental exposure, a 2-h time course was performed, revealing no statistical difference in movement until the 2-h mark (see Figure 2B). Longer experimental duration would likely result in an even greater differential in movement. Previous studies have quantified the adverse effect of hypoxia on swimming behavior19, ultimately compromising the integrity of the experimental results. Thus, it is hypothesized that hypoxia and/or CO2 production are the most likely causes of this deviation in SM compared to open-well controls. Unfortunately, the gas-tight seal that prevents the loss of volatile anesthetic from the enclosed well also prevents the diffusion of other gases into or out of the enclosed solution. This, of course, means restricted diffusion of CO2 and oxygen as they are respectively produced and consumed by the fish. This is an important limitation with respect to the length of experiment possible with this method. It is also important to note that with time, small bubbles begin to form in previously bubble-free wells, presumably due to metabolism. However, during testing, these bubbles did not hinder the tracking of the zebrafish by the software. Experiments of up to 1.5 h (plus equilibration time) appear to have no significant effect on the SM tracking of these wild-type zebrafish, but fish that exhibit more movement or have other increased metabolic demands may not be suitable for experiments of this length.
Volatile anesthetic concentration over time
To assure consistent concentration of volatile anesthetic over time, wells without zebrafish were used to measure a time course of anesthetic concentration as determined by HPLC. The same solution was added to each well, and the concentration of sevoflurane or isoflurane was measured every 15 min for 3 h (each measurement from a different well, as opposed to repeat measurements from the same well). As can be seen in Figure 3, virtually no anesthetic was lost throughout the time course with sevoflurane (vapor pressure of 197 mmHg at 25 °C), which has a lower vapor pressure than isoflurane (vapor pressure of 238 mmHg at 25 °C) which showed a 4% loss at 3 h. Neither volatile anesthetic showed appreciable loss at the 30-min to 60-min interval, which is typical of many behavioral experiments.
Potency of sevoflurane in larval zebrafish
This technique has been used to measure the potency of volatile anesthetics in zebrafish15, and may be superior to other methodologies previously used4. The median effective concentration (EC50) for sevoflurane by measures of spontaneous movement (SM) and elicited movement (EM) are shown in Figure 4. With the sealed-well technique, EC50 values of 62 µM (95% CI: 49-81) for SM, and 126 µM (95% CI: 81-193) for EM were determined. The SM value is comparable to the previously reported EC50 of 76 µM (95% CI: 50-114). However, the previously reported value of 240 µM (95% CI: 169-328) for EM is nearly double the value obtained here4. Without performing a similar HPLC study of this methodology (vacuum grease and a glass coverslip to create an air-tight seal), it cannot be said for certain if this difference is due to a loss of volatile anesthetic over time. However, it is a rational hypothesis, especially given that the stimulus for EM (acoustical tap) is recorded at the end of the behavioral observation time. Regardless of the method of sealing the well, any loss of sevoflurane concentration would have the greatest effect at later time points. Of course, these differences could be due to other underlying factors, such as the genetic background of the zebrafish.
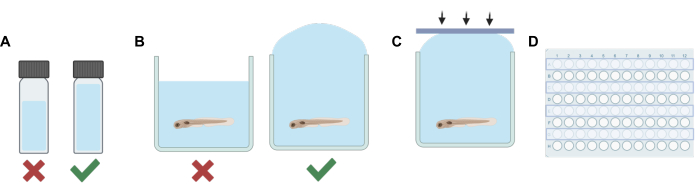
Figure 1: Key procedural steps. (A) When working with volatile solutions, dilution planning is done to minimize headspace in each vial. (B) Overfilling of wells is performed when adding experimental solutions to those containing zebrafish to eliminate air bubbles. (C) Silicone adhesive strips are applied from one side of the plate to the other to prevent air entrapment. (D) Every other row is used for experiments to prevent contamination of adjacent wells. Please click here to view a larger version of this figure.

Figure 2: Comparison of movement in sealed versus open wells. (A) Total spontaneous movement from 0 min to 4 min following a 15-min equilibration in the behavioral unit is compared. Movement of 5 days post-fertilization (dpf) zebrafish in open wells (254 mm, 95% CI: 185-322) and sealed wells (257 mm, 95% CI: 186-326) does not show a significant difference (P = 0.374). (B) Following a 15-min acclimation period in the zebrafish behavior system, fish (N ≥ 36) are observed for spontaneous movement (total distance traveled) over a 4-min period at the start of every half-hour. Data are shown as percent control (open wells), with statistical comparisons (ANOVA) made to the 0-h time point. Using a P-value threshold of <0.05, a significant difference in movement between open and sealed wells is observed at the 2-h mark (P = 0.0139), highlighted in red. Please click here to view a larger version of this figure.

Figure 3: Recovery of sevoflurane over time. The concentrations of sevoflurane and isoflurane in silicone adhesive-sealed glass well plates are determined by high-performance liquid chromatography (HPLC) over a 3-h period. Percent recovery, calculated as the average of three replicates, is shown in the lower portion of the graph. Isoflurane exhibits up to a 4% loss in concentration at 3 h, likely due to its higher vapor pressure (238 mmHg at 25 °C) compared to sevoflurane (197 mmHg at 25 °C). Please click here to view a larger version of this figure.
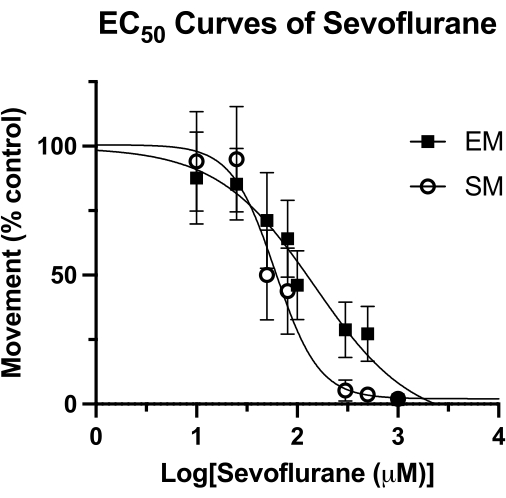
Figure 4: Sevoflurane EC50 curves. A sevoflurane EC50 curve in sealed wells is determined based on spontaneous movement (over a 4-minute period), and elicited movement (1 second period following an acoustic tap) of 5 dpf zebrafish. Movement is scaled to DMSO-only controls. The final EC50 value is calculated as 62 µM (95% CI: 49-81) for spontaneous movement (SM) and 126 µM (95% CI: 81-193) for elicited movement (EM). Please click here to view a larger version of this figure.
Discussion
This article describes a method for readily administering volatile anesthetics, like sevoflurane, to larval zebrafish, which was used to quantify volatile anesthetic potency in wild-type fish. Although this technique is conceptually simple, it can take practice to ensure an adequately sealed, bubble-free row of wells in a short time frame to minimize the loss of volatile agents. Because this method is intended for volatile compounds, it requires not only careful application of the silicone adhesive to the well plate but also careful preparation of experimental solutions to optimize the accuracy of volatile compound concentrations.
Entrapment of air can inhibit accurate visualization and tracking. In addition to practice sealing, careful filling and inspection of each well is critical as any bubbles present prior to sealing will be unlikely to be removed during the sealing process. Overfilling of wells also helps to prevent entrapment of air in each well. However, too much overfill can lead to the unintended transfer of fish into adjacent wells. If this happens, try to over-fill less. Minimizing excess over-fill will not only help the unintentional transfer of fish to adjacent wells, but may enhance the effectiveness of the adhesive used for sealing in the presence of large amounts of excess solution. Although the adhesive was specifically chosen in part for its ability to adhere to wet surfaces, starting with a clean and dry plate ensures that no other substances interfere with adhesion, thereby improving the overall integrity of the experimental setup. If bubbles still persist despite the lack of bubbles in well prior to adhesive application, optimization of overfilling, and silicone adhesive application technique, this may be due to the use of adhesive strips that are too narrow. Strips that are not wide enough may either not seal, or seal inadequately to prevent air from being introduced during or after the application process. Another potential introduction of error with this technique is the quality of the seal produced by the adhesive silicone strips, which can be caused by creases in the silicone, inadequate sealing of the adhesive (insufficient adhesive primer, cure time, or cleaning of surfaces prior to application of silicone). Although the adhesive itself appears to provide a sufficiently tight seal, without the silicone as a protective backing, the adhesive alone may fail to provide adequate water resistance to protect against the circulating water bath over the duration of an experiment.
Glass plates are well suited for this application as, unlike many plastics20, they are compatible with hydrophobic substances like volatile anesthetics, their surface is flat (unlike typical plastic well-plates), allowing for adhesion of the silicone strip, and have transparent bottoms (unlike Teflon and some plastic plates). Quartz well plates may provide increased optical clarity for experiments where higher-resolution imaging is warranted. All example data presented here were obtained in 96-well glass plates, and other similar plates are commercially available in larger well formats, but these were not tested by the authors. More solutions in a larger well may increase potential duration of an experiment with this technique due to greater availability of oxygen and may also facilitate experiments with older zebrafish, but modification of an experiment in this way would require further validation. Other modifications of this technique include the application of this technique to other behavioral paradigms (e.g., stimuli such as light)21, various genetic models, other types of pharmacologic studies (e.g., co-administration of drugs), and perhaps even adaptation for imaging studies.
This method provides a simple and accessible method for the administration of volatile compounds to zebrafish for the purpose of behavioral observation. Zebrafish are an increasingly popular model for the study of anesthetics, in particular intravenous (IV) anesthetics (propofol, etomidate, ketamine, etc.). This is in part because of the ease of drug administration, but there are also reduced pharmacokinetic considerations compared to the administration of IV anesthetics to non-aquatic animal models (worms, flies, rodents, humans, etc.) that require target-controlled infusion models for steady-state administration2. This is comparable to the administration of volatile agents in these animals, which is as simple as putting the animal in a box and having them breathe in the agent mixed with oxygen/air. The administration of volatile agents to aquatic animals (zebrafish, tadpoles) is more complicated since it requires a sealed aqueous environment. This differential tends to divide the use of certain animal models for certain anesthetics, but with the method presented here, both classes of anesthetics can be readily delivered to the same animal model. Being able to easily compare various experimental paradigms in a single model despite disparate routes of administration. This also could be adapted for the use of volatile anesthetics in a way that utilizes the other strengths of the zebrafish, including their genetic tractability, rapid development, and transparent embryos, which facilitate real-time observation of physiological responses. Understanding the impact of volatile anesthetics, such as isoflurane and sevoflurane, in zebrafish can enhance comprehension of their mechanisms of action and side effects. This novel methodology provides a robust framework for the investigation of volatile anesthetics in zebrafish.
Disclosures
The authors have no conflicts to disclose.
Acknowledgements
This work was funded by the Foundation for Anesthesia Education and Research (FAER). Figure 1 is created in BioRender.com.
Materials
| Name | Company | Catalog Number | Comments |
| 96 well glass microplates | Zinsser North America | 3600500 | "These glass microplates are made from special high purity, temperature resistant borosilicate glass and the surfaces are acid polished. Plates are rectangular in the standard SBS microplate footprint (85 x 127 mm).These glass reactor microplates are designed primarily for use with chemistry applications and are resistant to temperatures up to 530° C. They can be autoclaved. They are machined from a homogeneous glass sheet and feature uniform geometry and shape to provide consistent temperatures from well to well (within 0.2 degrees C typical)." |
| 2.0 mL Clear, Large Opening, 9 mm Thread Vials | Chemglass Life Sciences | CV-1150-1232 | "With a 40% larger opening, these vials are specifically designed to work in robotic arm auto samplers. They also incorporate the unique Step Vial design that precisely centers a limited volume insert in the neck of the vial. " |
| 3M Double Coated Tape 9490LE | AbbVie Inc | 24WG90 | "3M Double Coated Tape 9490LE with 3M Laminating Adhesive 300LSE provides high bond strength to most surfaces, including many low surface energy plastics such as polypropylene and powder coated paints. The acrylic adhesive also provides excellent adhesion to surfaces contaminated lightly with oil typically used with machine parts. 3M double coated tape 9490LE offers the added feature of 3M Laminating Adhesive 300MP on one side to provide excellent bond strength to a variety of foam and fabric materials (6 in x 5 yd)" |
| DanioVision Observation Chamber | Noldus | DVOC-0041 | "DanioVision is a complete system, designed for the high-throughput testing of zebrafish larvae in multi-well plates. It includes the Observation Chamber and renowned EthoVision XT video tracking software." |
| DanioVision Temperature Control Unit | Noldus | DVTCU-0011 | "The DanioVision Temperature Control Unit is a flow-through system: water flows evenly underneath the well plate at the temperature of your choice. The temperature is the same all throughout the well plate." |
| Ethovision XT16 software | Noldus | NSE-EV-BASE | "EthoVision XT is the most widely applied video tracking software that tracks and analyzes the behavior, movement, and activity of any animal, trademarked by Noldus" |
| Isoflurane | Piramal Critical Care | NDC # 66794-017-10 | Liquid for inhalation, a nonflammable nonexplosive inhalation anesthetic, containing 100 mL isoflurane. Stored at controlled room temperature 20º to 25º C |
| Scotch-Weld Instant Adhesive Primer AC79 Clear | 3M | "62-3916-0860-1 (Product ID) C2103N (Lot)" | "3M Scotch-Weld Instant Adhesive Primer AC79 is designed for use on difficult-to-bond low surface energy elastomers, such as EPDM, silicone and other rubbers. Surface preparation and application are straightforward and the adhesive primer is fast drying to keep jobs moving quickly." |
| Silicone Sheets 0.5 mm thick | various suppliers | N/A | translucent silicone sheets were purchased from multiple suppliers |
| Speedball Pop-in Hard Rubber Brayer with Plastic Frame, 4 Inches" | Speedball | 793728 | "The Speedball Pop-In Hard Brayer has a roller that snaps out for easy cleaning. Plastic, one piece frame with detachable 4 inch wide roller. Made from pure natural gum rubber with a ground finish. The hard rubber brayer is 4 inches wide with a sturdy plastic frame and great for glue application" |
| SureSTART 9 mm Screw Caps, Level 2 High-throughput Applications, Type: AVCS Screw Cap Black PP, White Silicone/RED PTFE Septa 1.0 mm | Thermo Scientific | 6ASC9ST1B | "Use Thermo Scientific SureSTART 9 mm Screw Caps with screw vials that have a 9 mm opening. The performance Level 2 caps are manufactured and tested to ensure low bleeding, robustness, and reproducibility of results for y routine GC-MS and LC-MS analyses. Choose from polypropylene caps with all-purpose silicone/PTFE septa of various hardness values designed to reduce autosampler needle issues. Our AVCS caps incorporate Advanced Vial Closure System technology to ensure optimal seal compression when closing a vial." |
| Ultane Sevoflurane | AbbVie Inc | NDC # 0074-4456-04 | Volatile Liquid for Inhalation, is packaged in amber colored bottles containing 250 mL sevoflurane. Stored at controlled room temperature 15º to 30º C |
| Wheaton Liquid Scintillation Vials, Caps Attached to Vials, Glass, Metal Foil / Pulp, 24-400, 20 mL | DWK Life Sciences | 986561 | 20 mL scintillation vials converted from Type I borosilicate glass tubing, PET, or HDPE. Available with cap attached. |
References
- Wasilczuk, A. Z., Maier, K. L., Kelz, M. B. The mouse as a model organism for assessing anesthetic sensitivity. Methods Enzymol. 602, 211-228 (2018).
- Shortal, B. P., et al. Development and validation of brain target-controlled infusion of propofol in mice. PLoS One. 13, 1-14 (2018).
- Halbach, K., et al. Yolk sac of zebrafish embryos as backpack for chemicals. Environ Sci Technol. 54 (16), 10159-10169 (2020).
- Bedell, V. M., Meng, Q. C., Pack, M. A., Eckenhoff, R. G. A vertebrate model to reveal neural substrates underlying the transitions between conscious and unconscious states. Sci Rep. 10, 1-10 (2020).
- Stetter, M. D., et al. Isoflurane anesthesia in amphibians: Comparison of five application methods. In American Association of Zoo Veterinarians. , Puerto Vallarta, Mexico. 255-257 (1996).
- Woll, K. A., Eckenhoff, R. G. High-throughput screening to identify anesthetic ligands using Xenopus laevis tadpoles. Methods Enzymol. 602, 177-187 (2018).
- Meyer, H. Zur Theorie der Alkoholnarkose. Arch Exp Pathol Pharmakol. 46, 338-346 (1901).
- Downes, H., Courogen, P. M. Contrasting effects of anesthetics in tadpole bioassays. J Pharmacol Exp Ther. 278, 284-296 (1996).
- Kalueff, A. V., et al. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish. 10, 70-86 (2013).
- Abramova, V., et al. Effects of pregnanolone glutamate and its metabolites on GABAA and NMDA receptors and zebrafish behavior. ACS Chem Neurosci. 14, 1870-1883 (2023).
- Germann, A. L., et al. Comparison of behavioral effects of GABAergic low-and high-efficacy neuroactive steroids in the zebrafish larvae assay. ACS Chem Neurosci. 15, 909-915 (2024).
- Hoyt, H., et al. Photomotor responses in zebrafish and electrophysiology reveal varying interactions of anesthetics targeting distinct sites on gamma-aminobutyric acid type A receptors. Anesthesiology. 137, 568(2022).
- Li, F., et al. Characterization of the locomotor activities of zebrafish larvae under the influence of various neuroactive drugs. Annals Trans Med. 6, 173(2018).
- McCarroll, M. N., Gendelev, L., Kinser, R. Zebrafish behavioural profiling identifies GABA and serotonin receptor ligands related to sedation and paradoxical excitation. Nat Commun. 10, 4078(2019).
- Plasencia, D. M., Rodgers, L. H., Knighton, A. R., Eckenhoff, R. G., White, E. R. Antagonism of propofol anesthesia by alkyl-fluorobenzene derivatives. Sci Rep. 14, 15943(2024).
- Dash, S. N., Lipika, P. Flight for fish in drug discovery: A review of zebrafish-based screening of molecules. Biology Lett. 19, 20220541(2023).
- Nelson, J. C., Michael, G. Zebrafish behavior as a gateway to nervous system assembly and plasticity. Development. 149, 177998(2022).
- Zhang, L., et al. Sevoflurane postconditioning ameliorates cerebral hypoxia/reoxygenation injury in zebrafish involving the Akt/GSK-3β pathway activation and the microtubule-associated protein 2 promotion. Biomed Pharmacother. 175, 116693(2024).
- Abdallah, S. J., Thomas, B. S., Jonz, M. G. Aquatic surface respiration and swimming behaviour in adult and developing zebrafish exposed to hypoxia. J Exp Biol. 218, 1777-1786 (2015).
- Targ, A. G., Yasuda, N., Eger, E. I. Solubility of I-653, sevoflurane, isoflurane, and halothane in plastics and rubber composing a conventional anesthetic circuit. Anesth Analg. 69, 218-225 (1989).
- Yang, X., et al. Drug-selective Anesthetic insensitivity of zebrafish lacking γ-aminobutyric acid Type A receptor β3 subunits. Anesthesiology. 131, 1276-1291 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved