Method Article
Generation and Characterization of Human Induced Pluripotent Stem Cell-derived Astrocytes Lacking Fragile X Messenger Ribonucleoprotein
In This Article
Summary
Here we describe a protocol that facilitates the differentiation of human induced pluripotent stem cells into functional forebrain-specific astrocytes. This enables investigations into the role of glial cells in the pathogenesis of neurodevelopmental disorders, such as Fragile X Syndrome, and modeling of other brain disorders.
Abstract
Fragile X syndrome (FXS), a leading inherited cause of autism spectrum disorder and intellectual disability, has been studied extensively using rodent models. More recently, human stem cell-derived model systems have also been used to gain mechanistic insights into the pathophysiology of FXS. However, these studies have focused almost exclusively on neurons. Further, despite growing evidence for a key role of glia in neuronal function in health and disease, little is known about how human astrocytes are affected by FXS.
Therefore, in this study, we successfully developed a protocol that captures key spatiotemporal milestones of brain development and aligns with the process of gliogenesis as well. Together this offers a useful framework for studying neurodevelopmental disorders. First, we patterned the human induced pluripotent stem cells into the neuroectodermal lineage with dual Suppressor of Mothers against Decapentaplegic (SMAD) inhibition and small molecules. Subsequently, we utilized specific growth factors and cytokines to generate control (CTRL) and FXS patient-derived astrocytic progenitor cells (APCs). Treatment of APCs with ciliary neurotrophic factor, a differentiating cytokine, regulated and drove the progenitor cells towards astrocytic maturation, yielding forebrain-specific glial fibrillary acidic protein-expressing astrocytes.
We found that these astrocytes are functional, as evidenced by their calcium responses to ATP application, and they exhibit dysregulated glycolytic and mitochondrial metabolism in FXS. Taken together, these findings provide a useful experimental platform of human origin for the investigation of cell-autonomous and non-cell-autonomous consequences of alterations in astrocytic function caused by neurodevelopmental disorders.
Introduction
Fragile X Syndrome (FXS), a common inherited form of intellectual disability and autism spectrum disorder (ASD), is caused by the lack of fragile X messenger ribonucleoprotein (FMRP) produced by the fragile X messenger ribonucleoprotein 1 (FMR1) gene (OMIM: #300624, https://www.omim.org/entry/300624). FMRP plays a role in the regulation of mRNA translation, mRNA granule formation and transport, and microRNA-mediated regulation of gene expression1. Thus, loss of FMRP impacts not just brain development but also adult brain function. Both mRNA transcript levels of FMR1 and immunostaining for FMRP in the brain have shown high neuronal expression, alongside significant expression in glial cells as well2. However, a vast majority of earlier studies in animal models of FXS focused primarily on neurons and aberrations in their function. Consequently, little is known about the role of glia in FXS3. Traditionally thought of as "passive support" cells4, there is accumulating evidence for astrocytes being critical in mediating a wide range of neuronal functions5,6, including promoting synaptogenesis7, refinement of developing neural circuits8, and neurotransmitter recycling9. In parallel, there is growing evidence for the role of astrocytes in disease pathogenesis and many neurological conditions have been associated with astrocytic dysfunction10.
While much of the earlier work using animal models of FXS focused on identifying and validating various molecular targets in neurons for treating FXS, these preclinical findings have not always led to successful clinical outcomes. Further, setbacks in recent clinical trials also underscore the need for human-based model systems. Models of neurological disorders based on human stem cell-derived brain cells offer a powerful strategy to bridge this gap between mechanistic insights from animal studies and limited success with clinical outcomes for patients. However, only a handful of these studies have focused on astrocytes and that too mostly on astrocytes that were spinal in origin. This, in turn, is relevant in light of studies showing that the structure and function of astrocytes vary between brain regions11,12. Thus, a better understanding of disease-induced changes in human astrocytes also needs to take into account these brain region-specific differences in astrocytes. However, models of neurodevelopmental disorders using human stem cell-derived astrocytes that are specific to the forebrain remain comparatively underexplored13. Hence, to begin to address these gaps, we describe protocols for generating forebrain-specific astrocytes from patient-derived induced pluripotent stem cells (iPSCs) carrying FXS mutations; further, we show that astrocytes are functional and display altered metabolism.
Protocol
All experiments using human induced pluripotent stem cells (hiPSCs) (Table 1) were performed after obtaining appropriate institutional regulatory approvals. Figure 1A represents complete differentiation protocol of hiPSCs to mature forebrain-specific astrocytes.
1. Maintenance and expansion of hiPSCs
- One day before plating the iPSCs, coat a 6-well dish with 1:60 diluted Matrigel (an Extracellular Matrix [ECM]) in Advanced Dulbecco's Modified Eagle Medium/Ham's F-12 (DMEM/F12) and store at 2-8 °C.
NOTE: Avoid storing Matrigel-coated dishes for >5 days at 2-8 °C, as this may cause protein degradation in the ECM over time. To avoid drying of the dish, coat a minimum of 1 mL of 1:60 diluted Matrigel per one well of a 6-well dish and spread evenly across the dish. If using the same day, keep the plate in a humidified incubator at 37 °C and 5% CO2 for 1 h. - On the day of culturing the hiPSCs, remove the coating material and add 1 mL of complete Essential 8 medium (E8 basal medium with Essential 8 supplement) along with the ROCK inhibitorat 1x final concentration and keep the dish in a 37 °C and 5% CO2 incubator before adding the hiPSCs.
- Resuspend the hiPSCs in complete E8 medium along with the ROCK inhibitor at 1x final concentration for better adherence of the colonies.
NOTE: Do not prepare large quantities of culture medium. Prepare only for 3-4 days maximum and store at 2-8 °C. - Next day, replenish the complete E8 medium without the ROCK inhibitor until the cells are 80% confluent (approximately 4-5 days).
- When they reach 80% confluency, detach the colonies enzymatically.
- To passage the iPSCs, remove the spent media from the dish and add 1 mL per well of prewarmed (37 °C) mixture of collagenase (2 mg/mL) and dispase (1 mg/mL) in a ratio of 1:1 and incubate at 37 °C for more than 20-30 min to allow the iPSC colonies to lift.
NOTE: Do not leave the dishes at 37 °C for more than 20-30 min; beyond this period, the colonies will disintegrate and cells will die.
- To passage the iPSCs, remove the spent media from the dish and add 1 mL per well of prewarmed (37 °C) mixture of collagenase (2 mg/mL) and dispase (1 mg/mL) in a ratio of 1:1 and incubate at 37 °C for more than 20-30 min to allow the iPSC colonies to lift.
- When the colonies start to lift, take the dishes out from the incubator and neutralize the enzyme activity by adding 2 mL of Dulbecco's Phosphate-Buffered Saline (DPBS).
- Scrape off the colonies with DPBS and collect the suspension in a 15 mL conical tube using a wide-bore 10 mL serological pipette.
- Gently triturate the suspension 2-3x to break up the colonies using a 10 mL serological pipette, and allow the colonies to settle down.
NOTE: Do not make the colonies into single cells; this may result in the cells not adhering to the dish and cause more cell death. Further, do not leave them as big colonies; this will lead to more differentiated colonies later. - Once the colonies settle down (approximately after 2 min), aspirate the DPBS enzyme mixture leaving approximately 1 mL in the tube.
- Add 2 mL of DPBS to the tube, mix the colonies by tapping, and allow them to settle. Repeat this 2x to remove all the residual enzyme from the colonies. Remove as much supernatant as possible after the 2nd wash, resuspend the colonies in 1 mL of complete E8 Medium, and plate in a freshly prepared dish as mentioned in steps 1.2-1.3.
- Alternatively, cryopreserve a proportion of colonies using 10% dimethyl sulfoxide (DMSO) as a cryoprotectant solution for future expansion and use.
- Prepare cryopreservation medium freshly by mixing 90% complete E8 medium and 10% DMSO as a cryoprotectant solution and place it at 2-8 °C until use.
- To cryopreserve, follow steps 1.5-1.10.
- During step 1.10, after the 2nd wash, allow the colonies to settle and remove the supernatant. To the colonies, add 1 mL of freshly prepared cold cryopreservation medium and transfer to cryovials.
NOTE: After adding the cryopreservation medium to the colonies, quickly transfer the contents to cryovials. Delaying may result in a lower revival rate as DMSO is a cryoprotectant that can damage cells. - Immediately move the cryovials to a cryobox and keep in a -80 °C freezer overnight.
- The next day, shift all the cryovials to a Liquid Nitrogen tank (LN2 tank) for future use.
NOTE: hiPSCs should be routinely karyotyped using G-banding (Supplemental Figure S1) for any abnormalities, characterized using immunocytochemistry for pluripotency (Figure 1B), and tested for mycoplasma.- To characterize hiPSCs using immunocytochemistry, plate the colonies on 1:60 Matrigel-coated, autoclaved 13 mm glass coverslips. Once they are 40% confluent, wash the cells with PBS-T (PBS-0.1% Tween 20), fix with 4% paraformaldehyde for 10 min, permeabilize with 0.3% Triton X-100 in PBS for 10 min, and block with 3% Bovine Serum Albumin (BSA) in PBS for 1 h to prevent non-specific binding.
- After blocking, incubate the cultures with primary antibodies for 1 h and then wash 3x with PBS-T for 5 min each, followed by corresponding secondary antibodies (Table of Materials) in the dark for 1 h.
- Mount the coverslips onto glass slides with mounting medium and acquire images by confocal laser scanning at 405 nm, 488 nm, 561 nm, and 633nm. Capture images at 512 x 512 pixels; set the Z step size at 0.5 µm with 1 airy unit of pinhole diameter.
2. Generation and characterization of astrocytic progenitor cells (APCs)
- Enzymatically lift the hiPSCs as mentioned in steps 1.5-1.10 and plate them onto a non-adherent suspension culture dish (100 mm) with chemically defined medium14 containing 50% Iscove's Modified Dulbecco's Medium (IMDM), 50% Ham's F-12 Nutrient Mix (F12), 5 mg/mL BSA, 1% Chemically Defined Lipid Concentrate (CD-Lipid), 450 µM Monothioglycerol, 7 µg/mL Insulin, 15 µg/mL Transferrin, 1% Penicillin-Streptomycin supplemented with forebrain patterning mitogens N-acetyl Cysteine (1 mM), LDN 193189 (0.1 µM), and SB431542 (10 µM) for 7 days.
NOTE: Small molecule inhibitors SB431542 and LDN-193189 (LDN) are inhibitors of bone morphogenetic protein and transforming growth factor-beta signaling pathways.
From this point onwards, the medium was replenished once in 2 days or following the Monday/Wednesday/Friday protocol. - Place the cell suspension on an orbital shaker at 40 rpm for 7 days under normoxic conditions to aid the development of corticospheres (Figure 1A).
- On day 8, transfer the corticospheres to a cell proliferation medium containing Advanced DMEM/F12 with 1% Antibiotic-Antimycotic, 1% N2 supplement, 1% glutamine substitute, 0.1% B27 supplement, and 2.5 ng/mL basic fibroblast growth factor (bFGF) for 7 days.
- Induce the spheres for glial specification by subjecting them to glial enrichment medium containing Advanced DMEM/F12 with 1% Antibiotic-Antimycotic, 1% N2, 1% glutamine substitute, 0.1% B27 supplement, 20 ng/mL epidermal growth factor (EGF), bFGF-H (20 ng/mL bFGF-5 mg/mL heparin) for 2 weeks to get early gliospheres (Figure 1A).
- For maturation of early gliospheres, replace bFGF-H with 20 ng/mL leukemia inhibitory factor (LIF) and maintain the spheres for 4 weeks.
- After 4 weeks in the maturation medium, maintain the spheres in glial enrichment medium for prolonged periods.To prevent aggregation and loss of viability, every 2 weeks, mechanically chop the gliospheres using a sterile industrial blade and replace the entire medium with DNase I to remove DNA fragments generated from chopping.
- Dissociate the gliospheres into monolayers of APCs using a Papain dissociation kit and plate onto a cell culture-treated adherent dish with 1:80 dilution of Matrigel coating.
- Propagate the APCs in glial enrichment medium until 80% confluent and enzymatically passage them using the enzyme cell detachment medium (see the Table of Materials).
- To passage:
- Remove the spent media and collect in a conical tube. To the cells, add the enzyme cell detachment medium and wait for 1-2 min. Once the cells begin to detach, add the spent media to neutralize the enzyme activity.
- Collect the cell suspension and centrifuge at 800 x ɡ for 2 min.
- Aspirate the supernatant, resuspend the cells in glial enrichment medium, and plate approximately 1 x 106 cells/well on to a 1:80 Matrigel-coated 6-well dish.
- For cryopreservation:
- Resuspend the cells in a cold mixture of 90% corticosphere proliferation medium (without bFGF) and 10% cryoprotectant. Transfer the resuspended cells into cryovials.
- Immediately move the cryovials to a cryobox and keep in -80 °C freezer overnight.
- The next day, shift all the cryovials to a Liquid Nitrogen tank (LN2 tank) for future use.
NOTE: APCs were cryopreserved using 10% DMSO until passage number 6.
- Characterize the dissociated APCs by immunostaining (as mentioned in steps 1.15.1-1.15.3) with vimentin and Nuclear Factor IA (NFIA) markers (Figure 1C,D) (See the Table of Materials for dilutions).
- To confirm forebrain regional specificity of hiPSC-derived APCs, test the cells for a forebrain marker such as human forkhead box G1 (hFOXG1) (positive) and hindbrain marker such as human Homeobox B4 (hHOXB4) (negative) using real-time qPCR (Figure 1E,F).
NOTE: The primer sequences used for these experiments have been listed in Supplemental Table S1.
3. Generation and characterization of a homogeneous population of forebrain-specific astrocytes
- Differentiation of astrocytes from APCs
- Use Astrocytic differentiation medium (ADM) for 14 days to differentiate APCs into astrocytes. Astrocytic differentiation medium contains Neurobasal, 1% Antibiotic-Antimycotic, 1% glutamine substitute, 1% N2 supplement, 0.2% B27 supplement, 1% non-essential amino acid medium (NEAA), and 10 ng/mL ciliary neurotrophic factor (CNTF).
- Confirm astrocytic identity by using immunostaining (as mentioned in steps 1.15.1-1.15.3.) with Glial fibrillary acidic protein (GFAP) and S100β expression markers (Figure 2A,B).
- De novo protein synthesis in APCs and astrocytes
- Maintain hiPSC-derived forebrain APCs and astrocytes on sterile autoclaved 13 mm glass coverslips and immunostain them as mentioned in steps 1.15.1-1.15.3.
- For de novo protein synthesis, use fluorescent non-canonical amino acid tagging (FUNCAT) method, explained briefly in steps 3.2.3-3.2.6 (Figure 3A).
- To ensure deficiency of methionine, remove the growth medium from the cultures and replace with methionine- and cysteine-free medium intermixed with 1 mM L-azidohomoalanine (AHA) for 30 min at 37 °C and 5% CO2.
- Wash the cultures with PBS-T, fix with 4% paraformaldehyde for 10 min, permeabilize with 0.3% Triton X-100 in PBS for 10 min, and block with 3% BSA in PBS for 1 h to prevent non-specific binding.
- Incubate the cultures in the dark for 1 h at room temperature with Click cell chemistry reaction mix and Alkyne Alexa Fluor 647, followed by primary antibodies for 1 h and corresponding secondary antibodies (Table of Materials) for 1 h (Figure 3B).
- Mount the coverslips onto glass slides with mounting medium and use them for further image analysis.
- Acquire images by confocal laser scanning at 405 nm, 488 nm, 561 nm, and 633nm.
- Capture images at 512 x 512 pixels; set the Z step size at 0.5 µm with 1 airy unit of pinhole diameter.
- Maintain the microscopy and imaging parameters at a constant setting across cell types. Capture images for each biological replicate in the same session.
- Perform intensity measurements using any standard image analysis software (e.g., Fiji or Imaris). In Imaris, use the SURFACES module to yield volumetric measurements of cell bodies positive for fluorescent non-canonical amino acid tagging (FUNCAT) signal with Vimentin (APCs) and GFAP (astrocytes) limiting the voxel range (250-350) to remove debris.
NOTE: Maintain the parameters across all biological replicates for both cell types.
- Adenosine 5′-triphosphate (ATP)-induced oscillatory calcium waves
- Plate astrocytes on a glass bottom dish (35 mm) at 5 × 103 cells/dish to measure cellular response to ATP.
- Allow the cells to adhere to the glass bottom for 24 h, wash them 3x with HBSS (20 mM HEPES, 137 mM NaCl, 5 mM KCl, 10 mM Glucose, 1 mM MgCl2, pH = 7.3) without calcium, and incubate in culture medium with 5 µM ratio metric dye Fura-2AM and 0.02% Pluronics F127 for 1 h at room temperature.
NOTE: Fura-2AM is a light- and temperature-sensitive chemical; store at -20 °C. - Post incubation, wash 2x with culture medium and replace with 2 mM Ca2+ containing HBSS and image the cells at 2 FPS speed using a 60x oil objective (1.35 NA) in a focus drift-compensating inverted microscope.
- Record ATP-induced calcium responses by bath application of ATP at the final concentration of 5 mM at 25th s (Figure 4A).
- Draw regions of interest around each cell using Fiji/ImageJ and calculate the F340/F380 ratio across all time points (Figure 4B).
- Cell metabolic assays
NOTE: The oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) of live cells were measured as per the manufacturer's protocol (see the Table of Materials).- One day prior to the assay, allow the astrocytes to adhere to the microplate (1.5 × 104 seeding density) in CNTF medium and incubate at 37 °C with 5% CO2.
- For the glycolysis stress assay:
- Replace the cell culture medium with base medium supplemented with 2 mM glutamine (pH adjusted to 7.4) and incubate in a non-CO2 chamber at 37 °C for 1 h.
NOTE: pH must be adjusted in a 37 °C water bath. - Insert the cartridge plate into the instrument to calibrate the sensor. Post calibration, replace the cartridge plate with the cell culture plate at a final concentration of the following kit components: 10 mM glucose, 1 µM oligomycin, and 50 mM 2-deoxy-glucose (2-DG) (Figure 5A).
- At the end of the test, lyse the cells and estimate total protein content.
- Replace the cell culture medium with base medium supplemented with 2 mM glutamine (pH adjusted to 7.4) and incubate in a non-CO2 chamber at 37 °C for 1 h.
- For the mitochondrion stress assay:
- Replace the CNTF medium with base medium supplemented with 2 mM glutamine, 1 mM pyruvate, 10 mM glucose (pH adjusted to 7.4) in a water bath at 37 °C.
- Incubate the plate in a non-CO2 chamber at 37 °C for 1 h. Meanwhile, insert the cartridge plate into the instrument to calibrate the sensor.
- Post calibration, replace the cartridge plate with the cell culture plate with a final concentration of the following kit components: 1.5 µM oligomycin, 1 µM carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone (FCCP), and 0.5 µM rotenone/antimycin A (Figure 5C).
- At the end of the test, lyse the cells and estimate total protein content.
Results
Human induced pluripotent stem cell (hiPSC) colonies were maintained using commercially available defined medium and immunostained for pluripotency markers, Oct4 and Nanog (Figure 1B). FXS and CTRL APCs showed comparable highly enriched proportions of cells immunopositive for vimentin and NFIA (Figure 1C,D). We found that APCs derived from healthy and FXS hiPSCs showed substantial upregulation of FOXG1 compared to HOXB4 (Figure 1F) consistent with a predominant forebrain identity. We observed a significant reduction in the number of GFAP-expressing astrocytes in FXS groups compared to CTRL astrocytes without affecting the number of S100β-expressing astrocytes (Figure 2B). Western blot analysis showed that the astrocytes generated from both FXS lines lacked expression of FMRP (Figure 2C,D). Quantification of newly synthesized proteins did not reveal any significant difference between CTRL versus FXS lines in either APCs or astrocytes (Figure 3C). However, we found protein synthesis to be consistently higher in APCs than in their respective astrocytes for each line (Figure 3C). These results suggest a stage-specific reduction in de novo protein synthesis from APCs to astrocytes in both FXS and CTRL lines.
Control, as well as FXS astrocytes, exhibited ATP-induced calcium transients (Figure 4A). However, a more detailed analysis revealed several key differences between the individual calcium transients recorded from CTRL, FXS1, and FXS2 astrocytes (Figure 4B). We classified the astrocytes as responders and non-responders to ATP based on the presence or absence of a calcium response, respectively (Figure 4C). The number of responders in FXS1 and FXS2 was significantly lower than CTRL. Specifically, we observed a significant reduction in the peak amplitude of the first calcium transient evoked after ATP application in both FXS astrocyte lines (Figure 4D). Further, the total duration of the first calcium transient was significantly shorter in FXS1 and FXS2 lines than in CTRL (Figure 4E). However, the number of calcium events (quantified as the total number of events per 250 s; Figure 4F), was comparable between lines. Taken together, these findings reveal alterations in calcium responses elicited by ATP in hiPSC-derived FXS astrocytes. ECAR results suggest a higher rate of glycolysis, glycolytic capacity, and glycolytic reserve in hiPSC-derived FXS astrocytes (Figure 5A,B). We found no significant difference in basal respiration in CTRL and FXS astrocytes. Cellular ATP production showed no significant difference between CTRL and FXS astrocytes. FCCP stimulation elicited significantly lower maximal respiration in FXS astrocytes than in CTRL astrocytes (Figure 5C,D).

Figure 1: Derivation of forebrain-specific astrocytic progenitor cells from hiPSCs. (A) Illustrative workflow for generation of astrocytes from hiPSCs. (B) Representative images of hiPSCs displaying comparable expression of Oct4/Nanog in CTRL and FXS lines. (C) Homogeneous population of hiPSC-derived APCs expressed a similar number of Vimentin- and NFIA-positive cells. (D) No significant difference in the expression of Vimentin- and NFIA across CTRL and FXS APCs. Statistical analysis was done using two-factor ANOVA followed by Tukey's pairwise comparison. (E) Schematic illustrating region-specific expression of FOXG1 and HOXB4 during development in vivo. (F) Graphical representation of FOXG1 expression compared with HOXB4 (using qRT-PCR) across genotypes suggesting propensity towards forebrain lineage. Statistical analysis by two-factor ANOVA followed by Sidak's multiple comparison test. For all experiments N = 3 biological replicates. ***p < 0.001. Scale bar = 50 µm. Error bars represent SEM. Abbreviations: hiPSCs = human induced pluripotent stem cells; CTRL = Control; FXS = Fragile X Syndrome; APCs = astrocyte progenitor cells. Please click here to view a larger version of this figure.

Figure 2: Reduced number of glial fibrillary acidic protein-positive hiPSC-derived FXS astrocytes. (A) Representative images of hiPSC-derived astrocytes exhibiting S100β and GFAP expression. (B) Percentage of GFAP-positive astrocytes was significantly lower in FXS-derived astrocytes compared to CTRL, although both exhibited similar S100β expression. Statistical significance determined by two-factor ANOVA followed by Tukey's pairwise comparison. (C) Immunoblot representing expression of FMRP in astrocytes from CTRL and FXS. (D) Graphs showing absence of FMRP in hiPSC-derived FXS astrocytes. Statistical analysis done by single-factor ANOVA with Tukey's pairwise comparison. For all the above experiments N = 3 biological replicates. Scale bar = 50 µm. ***p < 0.001. Error bars represent SEM. Abbreviations: hiPSCs = human induced pluripotent stem cells; CTRL = Control; FXS = Fragile X Syndrome; GFAP = glial fibrillary acidic protein; FMRP = Fragile X messenger ribonucleoprotein. Please click here to view a larger version of this figure.

Figure 3: Reduced protein synthesis due to differentiation from APCs to astrocytes. (A) Schematic workflow depicting labeling and visualization of protein synthesis in hiPSC-derived APCs and astrocytes using FUNCAT metabolic labeling. (B) Representative images of (top) Vimentin-positive hiPSC-derived APCs and (bottom) GFAP-positive astrocytes with FUNCAT/human Nuclei Antibody (hNA) label in CTRL and FXS-derived cells. (C) FUNCAT/volume of hiPSC-derived APCs was significantly higher than corresponding derived astrocytes. For all experiments N = 3 biological replicates. Scale bar = 50 µm. **p < 0.01, ***p < 0.001. Whiskers represent 1.5 × IQR. Abbreviations: hiPSCs = human induced pluripotent stem cells; CTRL = Control; FXS = Fragile X Syndrome; GFAP = glial fibrillary acidic protein; FUNCAT = fluorescent non-canonical amino acid tagging. Please click here to view a larger version of this figure.

Figure 4: Deficient Ca2+ signaling evoked by ATP in hiPSC-derived FXS astrocytes. (A) Representative traces of Ca2+ transients recorded from individual astrocytes upon external application of ATP at 25th s. (B) Averaged F340/F380 ratios depicting Ca2+ transients over 250 s after ATP application. (C) Grouped data showing higher percentage of non-responders to ATP in hiPSC-derived FXS astrocytes. Statistical significance determined by two-factor ANOVA with Tukey's pairwise comparison. (D-F) Quantification of first peak response (amplitude and duration) and frequency of events. (D) Shows a significant reduction in amplitude. Statistical significance determined by single-factor ANOVA with Tukey's pairwise comparison and (E) duration in hiPSC-derived FXS astrocytes. Statistical significance determined by Kruskal-Wallis test with Dunn's multiple comparison test; data set represented as mean ranks with SEM. (F) Graphical representation of Ca2+ transient frequency in hiPSC-derived FXS astrocytes. Statistical analysis by single-factor ANOVA with Tukey's pairwise comparison. For all experiments N = 3 biological replicates; n = 19 cells for each cell line. *p < 0.05, **p < 0.01, ***p < 0.001. Error bars represent SEM. # = Number. Abbreviations: hiPSCs = human induced pluripotent stem cells; CTRL = Control; FXS = Fragile X Syndrome; GFAP = glial fibrillary acidic protein. Please click here to view a larger version of this figure.
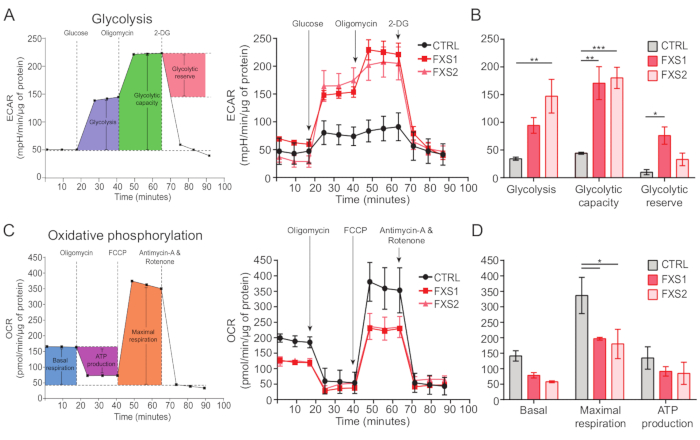
Figure 5: Bioenergetic deficits in hiPSC-derived FXS astrocytes. (A) Line graph representing extracellular acidification rate of hiPSC-derived astrocytes (CTRL and FXS) after addition of 10 mM Glucose, 1 µM Oligomycin, and 50 mM 2-DG sequentially and plotted on a line graph. (B) Glycolysis, glycolytic capacity, and glycolytic reserve quantification showed an increase in hiPSC-derived FXS astrocytes. Statistical analysis done by two-factor ANOVA with Tukey's pairwise comparison. (C) Line graph depicting oxygen consumption rate measurement by sequential addition of 1.5 µM Oligomycin, 1 µM FCCP, and 0.5 µM antimycin A and Rotenone. (D) Basal respiration, maximal respiration, and ATP production were quantified from the line graph and hiPSC-derived FXS astrocytes showed significant decrease in maximal respiration in comparison to CTRL astrocytes. Statistical analysis done by two-factor ANOVA with Tukey's pairwise comparison. For all experiments N = 2 biological replicates. *p < 0.05, **p < 0.01, ***p < 0.001. Error bars represent SEM. Abbreviations: hiPSCs = human induced pluripotent stem cells; CTRL = Control; FXS = Fragile X Syndrome; ECAR = extracellular acidification rate; 2-DG = 2-deoxyglucose; OCR = oxygen consumption rate; FCCP = carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone. Please click here to view a larger version of this figure.
| ID in manuscript | ID at source | Age | Sex | Reprogrammed cell line name | Reprogramming method | Starting cell type | G band karyotype |
| (years) | |||||||
| CTRL | ND30625 | 76 | M | CS25iCTR-18nxx | Episomal vectors | Fibroblast | Normal |
| FXS1 | GM07072 | 22 | M | CS072iFXS-n4 | Episomal vectors | Fibroblast | Normal |
| FXS2 | GM05848 | 4 | M | CS848iFXS-n5 | Episomal vectors | Fibroblast | Normal |
Table 1: Cell lines used in this studyto generate forebrain-specific astrocytes.
Supplemental Figure S1: Chromosomal analysis report. Normal GTG banding karyotype of (A) CTRL 46, XY, (B) FXS1 46, XY, and (C) FXS2 46, XY. Please click here to download this figure.
Supplemental Table S1: Primers used in this study for the characterization of cells. Please click here to download this table.
Discussion
Here, we describe a method to generate human iPSC-derived astrocytes that serve as an assay platform for characterizing functional changes induced by FXS. These astrocytes are functionally viable in culture and exhibit various properties, as evidenced by a range of measurements carried out in the present study. A critical step in this protocol is the initial conversion of iPSCs to corticospheres using the enzymatic lifting method. At this stage, optimizing the incubation time for collagenase type IV and dispase is crucial. If this is not optimized, it may cause the iPSCs to disintegrate or form single cells, which will prevent the formation of corticospheres. These corticospheres are patterned towards forebrain specificity using dual SMAD inhibition15 and are further differentiated into astrocyte progenitor cells (APCs). To achieve a high population of APCs, the mechanical chopping of gliospheres is crucial. Real-time qPCR can be used to confirm the forebrain-specificity; we observed a significant fold change increase in FOXG1, a marker for the forebrain, compared to HOXB4, a marker for the spinal cord.
APCs are then differentiated into astrocytes using CNTF, an activator of the JAK-STAT signaling pathway16. Results from this study indicate that terminally differentiated astrocytes derived from FXS iPSCs have lower GFAP levels, which is suggestive of impaired maturity. Although our analyses did not find any difference in de novo protein synthesis in FXS astrocytes and APCs compared to controls, we found a reduction in de novo protein synthesis in the transition from APCs to astrocytes in both FXS and control lines. The significant lowering in translation between APCs and astrocytes is consistent with a more streamlined and curated translation that has been shown for neurons versus neural precursor cells17. Earlier work that has examined aberrant protein synthesis has utilized whole brain tissues, neurons, and patient-derived fibroblasts, not astrocytes or APCs18. The only published reports on protein synthesis from patient-derived lines are not in astrocytes, but from lymphoblastoid cells19 and fibroblasts20,21. In light of these variations across studies, future studies would benefit from using FUNCAT measurements in astrocytes derived from additional FXS-patient-derived lines.
We assessed the functionality of astrocytes by measuring intracellular calcium dynamics22 and bioenergetics. FXS astrocytes showed reduced peak amplitude and duration of ATP-induced calcium transients, with fewer cells responding to ATP when compared to control astrocytes. Together, these alterations suggest that FXS disrupts calcium homeostasis in human astrocytes. These results are consistent with an earlier study23,24 reporting impaired IP3 receptor activity in FXS fibroblasts. Further, we observed increased glycolysis, glycolytic capacity, and glycolytic reserve in FXS astrocytes, along with reduced mitochondrial oxygen consumption rates.
The present study describes the generation and characterization of a new in vitro model for FXS astrocytes by leveraging the power of patient-derived iPSCs. Notably, it is only through an earlier study using human astrocytes co-cultured with human neurons that we uncovered the critical role of astrocytes determining the electrophysiological phenotype of the neurons14. Those studies, however, focused almost entirely on disease-induced changes in neuronal activity, and nothing was explored in astrocytes. Here, this protocol offers a novel framework for shifting our focus to functional alterations in astrocytes. This, in turn, will shed light on previously unexplored astrocyte-neuron interactions, such as glutamate signaling and electrical responses. Our results at this stage emphasize the need to prioritize astrocytes in future research.
It is critical to recognize the limitations of this protocol. First, it involves mechanical chopping, which leads to DNA fragmentation as well. This can cause the chopped gliospheres to clump, eventually leading to cell death. Second, the seeding density of APCs during the terminal differentiation step to astrocytes is essential to prevent contact mediated cell-cell inhibition. It is recommended to optimize this across cell lines to prevent detachment from the surface of the culture vessel.
Here, our findings are the first such analysis in human astrocytes as all past studies were based on other cell types such as lymphoblastoid cell lines and human fibroblasts25. Dysfunctional energy metabolism is a known player in the etiology of autism spectrum disorders as shown by the increased levels of human plasma lactate, significantly lower oxygen consumption rate in granulocytes26, and reduced expression of mitochondrial oxidative phosphorylation genes in the anterior cingulated, motor cortex, thalamus, and cerebellum of children with ASD27. Similar changes in energy metabolism are seen in neurodegenerative disorders like Parkinson's disease28 and Alzheimer's disease29, suggesting that increased glycolysis may compensate for reduced functional function30. Future studies are needed to explore if similar mechanisms are in play in dysfunctional energy metabolism seen in FXS patient-derived astrocytes.
At present, generating forebrain-specific astrocytes is a significant achievement, but future advancements could allow for the creation of region-specific glia, such as cortical/hippocampal astrocytes. This would provide deeper insights into the distinct roles played by these astrocytes in regional brain functions, synaptic plasticity, and neuronal-glial interactions. The ability to generate such region-specific astrocytes could advance models of neurodevelopment and neurodegenerative diseases. Ultimately, this could lead to more precise therapeutic strategies targeting astrocyte dysfunction in specific brain regions.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
We thank Prof. Sumantra Chattarji for intramural Funds. We thank Prof. Gaiti Hasan for access to the calcium imaging setup, Central Imaging and Flow Facility-National Centre for Biological Sciences, Padmanabh Singh and Prangya Hota for proofreading and suggestions, and the Labmate Asia team for their assistance in performing Seahorse XF assays.
Materials
| Name | Company | Catalog Number | Comments |
| 1-Thioglycerol | Sigma-Aldrich | M6145 | |
| Accutase solution | Sigma-Aldrich | A6964 | Enzyme cell detachment medium |
| Adenosine 5′-triphosphate magnesium salt | Sigma-Aldrich | A9187 | |
| Advanced DMEM/F-12 | ThermoFisher Scientific | 12634010 | |
| Antibiotic-Antimycotic (100x) | ThermoFisher Scientific | 15240062 | |
| B-27 Supplement (50x), serum-free | ThermoFisher Scientific | 17504044 | |
| Bovine Serum Albumin | Sigma-Aldrich | A9418 | |
| Chemically Defined Lipid Concentrate | ThermoFisher Scientific | 11905031 | |
| Collagenase, Type IV, powder | ThermoFisher Scientific | 17104019 | |
| Deoxyribonuclease I | Worthington Biochemical Corporation | LK003170 | |
| Dimethyl sulfoxide | Sigma-Aldrich | D2650 | |
| Dispase II, powder | ThermoFisher Scientific | 17105041 | |
| DPBS, no calcium, no magnesium | ThermoFisher Scientific | 14190144 | |
| Essential 8 Medium | ThermoFisher Scientific | A1517001 | |
| FXS1, FXS2 | Coriell Institute of Medical Research | GM07072, GM05848 | FXS patient cells |
| GlutaMAX Supplement | ThermoFisher Scientific | 35050061 | glutamine substitute |
| Ham's F-12 Nutrient Mix | ThermoFisher Scientific | 11765054 | |
| Healthy control cells | Cedars-Sinai Medical Center | ND30625 | healthy control cells |
| Heparin sodium salt from porcine intestinal mucosa | Sigma-Aldrich | H3149 | |
| IMDM | ThermoFisher Scientific | 12440053 | |
| Insulin, human | Roche | 11376497001 | |
| LDN 193189 | Stratech Scientific | S2618-SEL | |
| Leukemia Inhibitory Factor human | Sigma-Aldrich | L5283 | |
| Matrigel Growth Factor Reduced (GFR) Basement Membrane Matrix | Corning | 354230 | |
| MEM Non-Essential Amino Acids Solution (100x) | ThermoFisher Scientific | 11140050 | |
| Mouse FGF-basic (FGF-2/bFGF) Recombinant Protein | Peprotech | 450-33 | |
| Mr. Frosty freezing container | ThermoFisher Scientific | 5100-0001 | cryobox |
| N-2 Supplement (100x) | ThermoFisher Scientific | 17502048 | |
| N-Acetyl-L-cysteine | Sigma-Aldrich | A9165 | |
| Neurobasal Medium | ThermoFisher Scientific | 21103049 | |
| Nunc Biobanking and Cell Culture Cryogenic Tubes | ThermoFisher Scientific | 377267 | |
| Nunc Cell-Culture Treated 6 well dish | ThermoFisher Scientific | 140675 | |
| Papain Dissociation System | Worthington Biochemical Corporation | LK003150 | |
| Penicillin-Streptomycin | ThermoFisher Scientific | 15140122 | |
| Recombinant Human CNTF Protein, CF | R&D Systems | 257-NT-010 | |
| Recombinant Human EGF Protein, CF | R&D Systems | 236-EG-01M | |
| RevitaCell Supplement (100x) | ThermoFisher Scientific | A2644501 | |
| SB431542 | Tocris | 1614 | |
| Seahorse XFe24 Analyzer | Agilent Technologies | ||
| Seahorse XF Cell Mito Stress Test Kit | Agilent Technologies | 103015-100 | |
| Seahorse XF Glycolysis Stress Test Kit | Agilent Technologies | 103020-100 | |
| Tissue Culture Dishes-100 cm | Biostar Lifetech | TCD000100 | |
| Transferrin | Roche | 10652202001 | |
| VWR Razor Blades | VWR International | 55411-050 | |
| Antibodies | |||
| Primary antibody | Company | Catalog number | Dilution |
| Oct4 (C-10) | Santa Cruz Biotechnology | sc-5279 | Dilution: 1:250 Secondary antibody: Goat anti-Mouse IgG, Alexa Fluor 568 |
| Nanog | R & D Systems | AF1997 | Dilution: 1:100 Secondary antibody: Donkey anti-Goat IgG, Alexa Fluor 488 |
| Vimentin | Abcam | Ab5733 | Dilution: 1:500 Secondary antibody: Goat anti-Chicken IgY, Alexa Fluor 488 |
| NFIA | Abcam | Ab41851 | Dilution: 1:500 Secondary antibody: Goat anti-Rabbit IgG, Alexa Fluor 568 |
| GFAP-cy3 | Sigma | C9205 | Dilution: 1:500 Secondary antibody: NA |
| GFAP | DAKO | Z0334 | Dilution: 1:500 Secondary antibody: Goat anti-Rabbit IgG, Alexa Fluor 568 |
| S100β | DAKO | IR504 | Dilution: 1:500 Secondary antibody: Goat anti-Rabbit IgG, Alexa Fluor 488 |
| Anti-Nuclei Antibody, clone 235-1 | Merck Millipore | MAB1281 | Dilution: 1:1000 Secondary antibody: Goat anti-Mouse IgG1, Alexa Fluor 555 |
| Secondary antibodies | Dilution | ||
| Goat anti-Mouse IgG, Alexa Fluor 568 | Thermo Fisher Scientific | A11004 | 1:1000 |
| Donkey anti-Goat IgG, Alexa Fluor 488 | Thermo Fisher Scientific | A11055 | 1:1000 |
| Goat anti-Chicken IgY, Alexa Fluor 488 | Thermo Fisher Scientific | A11039 | 1:1000 |
| Goat anti-Rabbit IgG, Alexa Fluor 568 | Thermo Fisher Scientific | A11011 | 1:1000 |
| Goat anti-Rabbit IgG, Alexa Fluor 488 | Thermo Fisher Scientific | A11034 | 1:1000 |
| Goat anti-Mouse IgG1, Alexa Fluor 555 | Thermo Fisher Scientific | A21127 | 1:1000 |
References
- Santoro, M. R., Bray, S. M., Warren, S. T. Molecular of fragile X syndrome: A twenty-year perspective. Annu Rev Pathol. 7, 219-245 (2012).
- Wang, H., et al. Developmentally-programmed FMRP expression in oligodendrocytes: A potential role of FMRP in regulating translation in oligodendroglia progenitors. Hum Mol Genet. 13 (1), 79-89 (2004).
- Pacey, L. K. K., Doering, L. C. Developmental expression of FMRP in the astrocyte lineage: Implications for fragile X syndrome. Glia. 55 (15), 1601-1609 (2007).
- Zhang, Y., et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron. 89 (1), 37-53 (2016).
- Allen, N. J., Barres, B. A. Neuroscience: Glia - more than just brain glue. Nature. 457 (7230), 675-677 (2009).
- Khakh, B. S., McCarthy, K. D. Astrocyte calcium signaling: From observations to functions and the challenges therein. Cold Spring Harb Perspect Biol. 7 (4), a020404(2015).
- Allen, N. J., et al. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature. 486 (7403), 410-414 (2012).
- Chung, K., et al. Structural and molecular interrogation of intact biological systems. Nature. 497 (7449), 332-337 (2013).
- Rothstein, J. D., et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 16 (3), 675-686 (1996).
- Almad, A., Maragakis, N. J. A stocked toolbox for understanding the role of astrocytes in disease. Nat Rev Neurol. 14 (6), 351-362 (2018).
- Zhang, Y., Barres, B. A. Astrocyte heterogeneity: An underappreciated topic in neurobiology. Curr Opin Neurobiol. 20 (5), 588-594 (2010).
- Clarke, L. E., Barres, B. A. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 14 (5), 311-321 (2013).
- Bradley, R. A., et al. Regionally specified human pluripotent stem cell-derived astrocytes exhibit different molecular signatures and functional properties. Development. 146 (13), dev170910(2019).
- Das Sharma, S., et al. Astrocytes mediate cell non-autonomous correction of aberrant firing in human FXS neurons. Cell Rep. 42 (4), 112344(2023).
- Chambers, S. M., et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 27 (3), 275-280 (2009).
- Hu, X., et al. The JAK/STAT signaling pathway: from bench to clinic. Sig Transduct Target Ther. 6, 402(2021).
- Baser, A., et al. Onset of differentiation is post-transcriptionally controlled in adult neural stem cells. Nature. 566 (7742), 100-104 (2019).
- Pal, R., Bhattacharya, A. Modelling protein synthesis as a biomarker in fragile x syndrome patient-derived cells. Brain Sci. 9 (3), 1-12 (2019).
- Gross, C., Bassell, G. J. Excess protein synthesis in FXS patient lymphoblastoid cells can be rescued with a p110β-selective inhibitor. J Mol Med. 18 (3), 336-345 (2012).
- Kumari, D., et al. Identification of fragile X syndrome specific molecular markers in human fibroblasts: A useful model to test the efficacy of therapeutic drugs. Hum Mutat. 35 (12), 1485-1494 (2014).
- Jacquemont, S., et al. Protein synthesis levels are increased in a subset of individuals with fragile X syndrome. Hum Mol Genet. 27 (12), 2039-2051 (2018).
- Bowser, D. N., Khakh, B. S. ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks. J Neurosci. 24 (39), 8606-8620 (2004).
- Schmunk, G., Boubion, B. J., Smith, I. F., Parker, I., Gargus, J. J. Shared functional defect in IP3R-mediated calcium signaling in diverse monogenic autism syndromes. Transl Psychiatry. 5 (9), e643-e710 (2015).
- Peteri, U. K., et al. Generation of the human pluripotent stem-cell-derived astrocyte model with forebrain identity. Brain Sci. 11 (2), 209(2021).
- Alvarez-Mora, M. I., et al. Impaired mitochondrial function and dynamics in the pathogenesis of FXTAS. Mol Neurobiol. 54 (9), 6896-6902 (2017).
- Giulivi, C., et al. Mitochondrial dysfunction in autism. JAMA. 304 (21), 2389-2396 (2010).
- Anitha, A., et al. Brain region-specific altered expression and association of mitochondria-related genes in autism. Mol Autism. 3 (1), 12(2012).
- Teves, J. M. Y., et al. Parkinson's disease skin fibroblasts display signature alterations in growth, redox homeostasis, mitochondrial function, and autophagy. Front Neurosci. 11, 737(2018).
- Yao, J., et al. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 106 (34), 14670-14675 (2009).
- Sonntag, K. C., et al. Late-onset Alzheimer's disease is associated with inherent changes in bioenergetics profiles. Sci Rep. 7 (1), 14038(2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved