Method Article
A Unique Mouse Model for Quantitative Assessment of Biofilm Formation on Surgical Implants in Subcutaneous Abscess
* These authors contributed equally
In This Article
Summary
This protocol describes a unique experimental model of implant-related infections that enables the simultaneous incubation of two implants with bacteria under identical conditions within a single mouse. It also allows precise assessment of biofilm formation on implant surfaces using optimized comparative analytical methods, demonstrating advanced techniques for evaluating biomaterials' antimicrobial properties.
Abstract
To develop a novel biomaterial with antibacterial properties for orthopedic surgical procedures, establishing an experimental animal model of implant-related infections that closely mimics the pathological state is crucial. Additionally, a quantitative comparison with control samples is required to assess biofilm formation on materials. However, current animal models, which involve implanting each individual with a single material, may yield inconsistent outcomes due to the heterogeneity of infection status among subjects. Furthermore, accurately quantifying biofilm formation on materials in vivo remains challenging, and the findings may lack reliability. To address these issues, this study demonstrated a unique mouse model of implant-related infection that enables the simultaneous incubation of two implants with bacteria in an enclosed environment within a single mouse, forming an encapsulated subcutaneous abscess. A mature air pouch was initially created beneath the skin of the back. Two stainless steel wires were connected and placed into the pouch, followed by the inoculation of Xen 36, a bioluminescent strain of Staphylococcus aureus. By 14 days after inoculation, a subcutaneous abscess had formed around the wires. The biofilm was completely removed from the surface of each wire, and the dissolved bacterial suspensions were accurately measured using optimized methods to assess biofilm formation on the implant, determine colony-forming units, and perform quantitative polymerase chain reaction analysis. By leveraging the lux operon of the bioluminescent bacteria, the relative expression levels of luxA and 16S rRNA were used to determine the bacterial load within the biofilm on each wire. This optimized comparative analytical approach enables precise assessments of biofilm formation on two wires under uniform infection conditions within a single mouse model and may facilitate the advancement of biomaterials with antibacterial properties.
Introduction
Implant-related infections remain a significant challenge in clinical settings because they may cause high rates of morbidity and mortality in orthopedic surgery despite advances in surgical technique and implant design1. Although the incidence of infections associated with surgical implants has significantly decreased due to modern standards of aseptic control in the operating room environment and appropriate protocols for peri-operative antibiotic prophylaxis, the incidence of implant-related infections in primary surgery remains 2%-5%2.
The primary underlying mechanism of implant-related infections is the formation of a biofilm on the implant surfaces, which protects microorganisms from antibiotics and the immune system, making the infection difficult to eradicate3,4. As bacterial adhesion onto the implant surface is crucial during the first stage of biofilm formation, minimizing bacterial adhesion and subsequent biofilm formation is an essential strategy to reduce the risk of implant-related infections. Despite the antibacterial properties of various implant technologies, these have not been widely used clinically due to adverse events, such as cell toxicity and allergies5,6,7,8. Therefore, there is still an unmet clinical need for antibacterial implants that harmonize safety, efficacy, stability, and durability to reduce the risk of implant-related infections. The research and development of implants with antibacterial properties could advance surgical technology to overcome these problems.
Assessing the antibacterial properties of various biomaterials using small animal models is essential prior to proceeding to larger animal models and clinical trials9. Numerous studies have shown applicable mouse models of implant-related infections using a bioluminescent bacterium, which contains the luxABCDE operon10,11,12,13,14,15. While these models accelerate research into developing antibacterial implants or technologies, they have certain limitations. First, advanced expertise and specialized equipment, such as X-rays or dedicated imaging systems, are often required to assess the bacterial load on the implants placed in mouse models directly and accurately. Second, while collected implants typically evaluate a solitary implant at a single infection site per animal, the infection conditions and immunological responses may vary among individuals, potentially leading to variability in the outcomes of comparative assessments. Therefore, when comparing the antibacterial effects of various biomaterials in vivo, implanting and inoculating them with bacteria in uniform settings is more beneficial to address these issues. Additionally, it is essential to optimize the current methodology and conduct quantitative assessments with reproducibility and accuracy by exploiting the characteristics of the animal model and the bacteria used.
This study presents a novel experimental approach for in vivo implant-related infections that enables precise measurements to assess the biofilm formation on the surfaces of two implants within a single mouse model through comparative inter- and intra-individual analytical methods. The biofilm on each implant can be quantified using optimized methods for visualizing the biofilm on the implant, determining the colony forming units (CFU) and quantitative polymerase chain reaction (qPCR) analysis of a bioluminescent strain of Staphylococcus aureus, Xen 36. The previous study showed that a novel metal implant possesses promising in vivo antibacterial efficacy against Staphylococcus aureus using this comprehensive approach16. This methodology can be easily implemented in a standard laboratory setting and may accelerate research into developing antibacterial biomaterials.
Protocol
All animal procedures are approved by the Institutional Animal Care and Use Committee (IACUC) at the University of California San Francisco (UCSF) and are performed in a BSL2 facility after consultation with and approval by the UCSF Biosafety Hazard Program, administrated by UCSF Environmental Health and Safety. Male and female C57BL/6 mice (12-16 weeks old, 25-50 mg) were used. The details of the reagents and the equipment used are listed in the Table of Materials.
1. Bacteria preparation
- Use the bioluminescent Staphylococcus aureus Xen36 (ATCC49525) as the pathogen.
NOTE: This strain is obtained from the American Type Culture Collection (Manassas, VA) and uniquely utilizes a luxABCDE operon, which is optimized and integrated into the host's native plasmid. Xen36 also uses the kanamycin resistance gene linked to the lux operon. - Add a small amount of frozen glycerol stock of Xen 36 and add the pieces to 5 mL of tryptic soy broth (TSB) medium containing 200 µg/mL kanamycin.
- Incubate the culture overnight at 37 °C in a shaking incubator (200 rpm).
- Streak Xen36 onto TSB agar plates (TSB in agar 1.5%) containing 200 µg/mL kanamycin and incubate overnight at 37 °C.
- Isolate single colonies of Xen36 and culture in 5 mL of TSB containing 200 µg/mL kanamycin overnight at 37 °C in a shaking incubator (200 rpm).
- Measure the absorbance of the resultant culture at 630 nm.
- Dilute the culture and make a Xen36 culture of 1.0 x 108 CFU/mL based on the absorbance at 630 nm.
NOTE: An absorbance measurement at 630 nm (against a TSB blank) of 0.5 is roughly equivalent to 1.0 x 108 CFU/ml of Xen36. - Dilute the culture and make a Xen36 culture of 1.0 x 105 CFU/mL.
2. Preparation of connected implants
- Use two rod-shaped wires composed of commercially available surgical-grade stainless steel (SUS316L), each measuring 8 mm in length and 0.5 mm in diameter.
- Connect the two wires vertically by inserting them into both ends of a 20 µL pipette tip cut to a length of approximately 3 mm.
NOTE: Insert the vertically connected wires into the tip of the 18 G needle (Figure 1). Ensure that the wires are firmly secured by the pipette tip to prevent disconnection. - Autoclave the wires before surgically implanting them into the mice.
3. Creation of a mature subcutaneous pouch
NOTE: 7 days before bacterial inoculation, create the air pouch as follows:
- Anesthetize the mouse (C57BL/6) with 2% isoflurane via inhalation (following institutionally approved protocols).
NOTE: Assess the appropriate level of anesthesia by observing the respiratory rate, muscle tone, toe pinch, corneal reflex, and color of the mucous membranes. Use ophthalmic ointment on eyes to prevent dryness while under anesthesia. - Transfer the anesthetized mouse to the surgical bed and shave the mouse's dorsal cervical/thoracic region.
- Swab and sterilize the entire region with 70% ethanol and povidone-iodine.
- Fill a 10 mL syringe with sterile air and attach a 27 G needle to the outlet.
- Gently pinch and elevate the base of the mouse's neck to create space between the subcutaneous tissue and the fascia.
- Place the needle into the midline between the mouse's scapulae and inject 3 mL of sterile air subcutaneously to create the air pouch (Figure 2).
NOTE: While injecting air, hold down both sides of the back with the opposite hand to ensure the air spreads to the center of the back, creating a pouch in the middle. - Return the mouse to a cage warmed with a thermal pad and monitor it closely until it has recovered from the anesthesia.
- Inject 3 mL of sterile air as described above to maintain the cavity's inflation and create a mature pouch every 2 days.
NOTE: Before each inflation, aspirate the air from the pouch to confirm the proper placement of the needle tip. Carefully remove the syringe from the needle, leaving the needle tip in the pouch. Reinflate the pouch with 3 mL of sterile air.
4. Implantation of connected wires and bacterial inoculation
- 7 days after the first air injection, anesthetize the mouse as described in step 3.1.
NOTE: During the surgical procedure, constantly check that the mouse is breathing and is anesthetized. The entire surgical procedure usually takes 10-15 min when performed by a trained surgeon. Anesthesia is maintained by placing a tube delivering 2% isoflurane mixed with oxygen adjacent to the mouse snout. - Swab and sterilize the entire region with 70% ethanol and povidone-iodine.
- Inject bupivacaine subcutaneously just before surgery.
- Make a 3 mm midline-longitudinal incision at the top of the pouch.
- Insert an 18 G needle containing the connected wires into the pouch through the hole and push out the wires using an inner syringe of a 25 G spinal needle (Figure 3A,B).
- Leaving the tip of the 18 G needle inside the pouch, gently remove the inner cylinder and inject 3 mL of 1.0 x 105 CFU/mL Xen36 culture using the syringe (Figure 3C,D).
- Carefully remove all needles, close the skin using a wound clip, and seal with topical skin adhesive (Figure 3E,F).
- Ensure there is no leakage, return the mouse to the individual cage warmed with a thermal pad, and monitor as before.
5. Extraction of the implants from the subcutaneous abscess
- After euthanasia via overdose of isofluorane, shave the dorsal cervical, thoracic, and lumbar region of the mouse, and swab and sterilize the entire region with 70% ethanol and povidone-iodine (Figure 4A).
NOTE: The euthanasia was conducted in accordance with the American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals. - Make a 2 cm midline incision in the lumbar region and carefully exfoliate the subcutaneous tissue using scissors.
- Extend the incision and subcutaneous dissection proximally and separate the adherent tissue surrounding the abscess (Figure 4B - D).
- Excise the entire subcutaneous abscess (Figure 4E).
- Cut the abscess and carefully extract the wire from the inside of the abscess (Figure 4F).
6. Quantification of the formed biofilm on the implant surfaces
- Crystal violet assay
- Place the wires extracted from the abscess into individual wells of a 24-well plate containing 1 mL of deionized (DI) water.
- Transfer the wires to new wells containing 1 mL of DI water to remove any loosely adherent bacteria from the surface of the wires.
- Fix the biofilm on the wires with 100% ethanol for 1 min and allow them to dry.
- Transfer the wires to new wells containing 1 mL of 0.1% crystal violet reagent.
- After 15 min of staining, gently wash the wires twice with DI water to remove any excess dye.
- Place the implant into a 1.5 mL microcentrifuge tube containing 250 µL of 33% acetic acid for 15 min; solubilize the crystal violet adherent to the biofilm on the implant, followed by vortexing for 1 min.
- Transfer 200 µL of the suspension to a 96-well plate and measure the absorbance at OD630 nm using a microplate reader. All measurement is performed in triplicate.
- Colony forming units (CFU) counting
- Remove any loosely adherent bacteria from the surface of the wires as described in step 6.1.2.
- Place the implant into a 1.5 mL microcentrifuge tube containing 200 µL of 10x trypsin and incubate at 37 °C for 1 h.
- Vortex for 1 min and sonicate in a water bath at 100 W for 5 min, followed by additional vortexing for 30 s to detach the biofilm into the suspension.
- Inoculate 10 µL of the serially diluted suspension onto the TSB agar plates (TSB in agar 1.5%) with 200 µg/mL kanamycin.
NOTE: Perform this procedure in triplicate for each solution. - After incubating the plates at 37 °C for 24 h, count the colonies on the plates and calculate the number of bacterial cells in the original culture using the mean colony counts17.
NOTE: To ensure that all bacteria are removed from the wires, which is crucial for accurate measurement, stain the wires with crystal violet and observe them after applying the suspension to the plates. In the preliminary experiments, the combination of the above mechanical and chemical dissociation successfully removed all Xen36 biofilms from the surfaces of the conventional stainless steel wire (Figure 5).
- Quantitative polymerase chain reaction (qPCR) analysis
- Remove any loosely adherent bacteria from the surface of the wires as described in step 6.1.2.
- Place the implant into a 1.5 mL microtube containing 600 µL of TSB.
- Extract DNA using the pellet-free modified alkaline lysis system16.
NOTE: The final volume of eluted DNA is 20 µL. - Dilute the DNA 1:10 and store it at -20 °C.
- Perform qPCR for the luxA and 16S rRNA genes16.
NOTE: All qPCR reactions are performed in triplicate. The total reaction mix volume is 10 µL, containing 3 µL of DNA template, 5 µl of SYBR Green, 1 µL of nuclease-free water, and 0.5 µL of each primer. Primers for the luxA gene are 5'- GAGCATCATTTCACGGAGTTTG -3' and 5'- ATAGCGGCAGTTCCTACATTC -3'. Primers for the 16S rRNA gene are 5'- GTGGAGGGTCATTGGAAACT - 3' and 5'- CACTGGTGTTCCTCCATATCTC - 3'. The PCR conditions are as follows: initial denaturation for 2 min at 94 °C followed by 40 cycles of 15 s at 94 °C, 30 s at 60 °C, and 30 s at 72 °C, and a final extension at 72 °C for 5 min. - Plot the mean of the three-cycle threshold (Ct) values is plotted against a calibration curve generated with DNA purified directly from serially diluted pure Xen36 cultures to estimate the bacterial load on the implant (Figure 6).
Results
This study evaluated the reliability of a comprehensive approach using a novel mouse model of implant-related infection with optimized quantitative assessments of biofilm formation on implant surfaces, which has been used in the previous study16. The two identical implants were used to examine the biofilm formed on their surfaces, aiming to verify that both implants could be incubated simultaneously in uniform infection conditions within a solitary subcutaneous space in a single mouse model. Mice underwent a subcutaneous pouch creation procedure (Figure 2), and after 7 days, the connected stainless steel wires were implanted into the mature pouch, followed by inoculation with Staphylococcus aureus Xen36 (Figure 3). By 14-day post-inoculation, an encapsulated abscess of approximately 2 cm in length and 1 cm in width, containing the connected wires formed in the pouch (Figure 4). The wires were harvested from the abscess, and the biofilm formed on each wire was assessed by crystal violet staining, CFU counting, and qPCR analysis. The crystal violet-stained wires showed consistent biofilm formation on the surfaces, with no observable changes between both wires (Figure 7A). The precise measurements to assess the biofilm formation through comparative inter- and intra-individual analytical methods indicated that the absorbance measurements of dissolved crystal violet assay (Figure 7B), CFU counting (Figure 7C), and qPCR analysis (Figure 7D) showed no statistically significant differences in bacterial load within the biofilm on both wires. These results suggested that both wires were simultaneously incubated under identical infection conditions at a solitary subcutaneous pouch within a single mouse model.
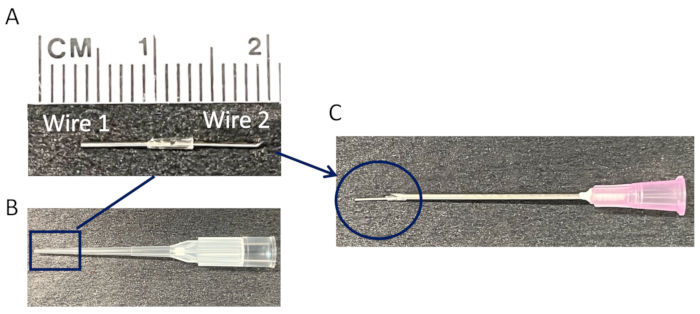
Figure 1: Preparation of connected wires for subcutaneous pouch implantation. (A) A representative image of a single implant consisting of two connected stainless steel wires (length: 8 mm, diameter: 0.5 mm). (B) A tip of a 20 µL pipette tip serves as a connection for the two wires. (C) The connected wires are subsequently inserted into the tip of an 18 G needle for implantation into a mature subcutaneous pouch. Please click here to view a larger version of this figure.

Figure 2: Creation of a mature subcutaneous pouch for implantation and inoculation. (A) The lateral view, and (B) the top view of a fully formed subcutaneous pouch just after air injection. A subcutaneous pouch is created on the back of a mouse model by inserting a 27 G needle into the skin along the midline between the scapulae and injecting 3 mL of sterile air subcutaneously. Thereafter, 3 mL of sterile air is administered every other day for 7 days to facilitate the maturation of a subcutaneous pouch. Please click here to view a larger version of this figure.

Figure 3: Implantation of connected wires and bacterial inoculation into a mature subcutaneous pouch. (A) The lateral view, and (B) the top view of the needle insertion procedure into a mature subcutaneous pouch. The connected wires are placed into the tip of an 18 G needle, and an inner syringe of a 25 G spinal needle is used to push out the wires. They are inserted into the mature subcutaneous pouch through the skin hole. The wires are subsequently placed into the pouch using a 25 G spinal needle. Bacterial inoculation into the pouch as observed from the lateral view (C) and the top view (D). After attaching a syringe containing bacterial solution to the 18 G needle, 3 mL of Xen36 culture (1.0 x 105 CFU/ml) is inoculated into the pouch. The insertion site is closed after removing the 18 G needle, with no leakage detected from either the lateral view (E) or the top view (F). Please click here to view a larger version of this figure.

Figure 4: Collection of connected wires in a subcutaneous abscess. (A) A lateral view of a mouse model for implant-related infections at 14-day post-inoculation. (B) The lateral view, (C) the top view, and (D) the bottom view of the subcutaneous abscess with surrounding well-developed blood vessels after separating the adherent tissues. (E) The subcutaneous abscess is entirely encapsulated, and (F) the connected wires are fully enveloped within it. Please click here to view a larger version of this figure.
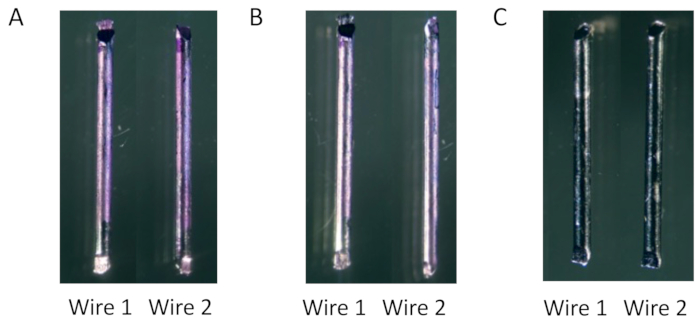
Figure 5: Removal of biofilm formed on wires for CFU assessment. (A) The biofilm formed on the surfaces of each wire is consistently stained with crystal violet at 14 days post-inoculation in vitro. (B) The stained biofilm is diminished by vortexing and sonication. (C) It is entirely eliminated by adding trypsin treatment. Please click here to view a larger version of this figure.

Figure 6: Calibration curve of 16S rRNA and luxA genes in Staphylococcus aureus. A calibration curve converts Ct values to the equivalent bacterial load in CFU. A serial dilution of pure Staphylococcus aureus Xen36 culture (108 to 101 CFU) is created. A high Pearson correlation coefficient is obtained for 16S rRNA (R2 = 0.951) and luxA (R2 = 0.985), indicating linear standard curves. Please click here to view a larger version of this figure.

Figure 7: Quantitative assessments of biofilm formation on connected wires within a mouse model. (A) Representative images of a single implant consisting of two connected stainless steel wires for the subcutaneous pouch implantation (left) and crystal violet-stained biofilm formed on the surfaces of each wire (Wire 1 or Wire 2) at 14-day post-inoculation (right). Measurements to assess the biofilm using comparative inter- and intra-individual analytical methods are performed for each wire within a mouse model (total n = 12), including (B) the absorbance measurement of crystal violet-stained biofilm (n = 4; circle dot indicates Mouse #1; triangle dot indicates Mouse #2; square dot indicates Mouse #3; diamond dot indicates Mouse #4). (C) CFU counting (n = 4; circle dot indicates Mouse #5; triangle dot indicates Mouse #6; square dot indicates Mouse #7; diamond dot indicates Mouse #8), and (D) qPCR analysis (n = 4; circle dot indicates Mouse #9; triangle dot indicates Mouse #10; square dot indicates Mouse #11; diamond dot indicates Mouse #12). The differences between the wire groups are assessed using a one-way analysis of variance (ANOVA). All data are presented as mean ± standard error. Statistically significant values were defined as p < 0.05. Please click here to view a larger version of this figure.
Discussion
This study demonstrated a comprehensive approach for precise quantitative assessments of biofilm formation on multiple implants by leveraging a novel mouse model and optimized analysis techniques for implant-related infection. To ensure that two implants were incubated under identical infection conditions in the same subject, a mature subcutaneous pouch was created within a single mouse through repeated air injections. This model provided a closed and stable environment where two connected implants could be incubated under identical infection conditions, even as the mouse moved. Following inoculation with Staphylococcus aureus, a solitary encapsulated subcutaneous abscess, approximately 2 cm in length, developed, fully enclosing both wires and closely resembling the characteristics of clinical implant-related infections. Both wires were extracted from the abscess, and the biofilm was removed from each surface using mechanical and chemical dissociation. The dissolved bacterial solution was utilized to quantitatively analyze the bacterial load within the biofilm on each wire. A comparative inter- and intra-individual analytical analysis of multiple wires was conducted between the two types of wire groups for quantitative assessment. Additionally, both wires placed within the same mouse were comparatively measured for more accurate evaluation because they were simultaneously incubated with a uniform infection. The qPCR analysis used the relative expression levels of the 16S rRNA gene and the lux operon, which encodes luciferase in a bioluminescent Staphylococcus aureus, for quantitative assessment. This study involving two identical wires resulted in no significant differences in all quantitative assessments of biofilm formation between the wire groups in several mice or between wires within a single mouse. Meanwhile, the previous study showed significant differences in quantitative evaluations of biofilm formation between two distinct wire groups in several mice16. Therefore, this comprehensive approach optimizes the mouse model and analytical techniques to minimize the variability of outcomes, potentially enhancing the accuracy and reproducibility of quantitative assessments of biofilm formation on multiple biomaterials.
Several in vivo models of implant-related infections have been established to assess the antimicrobial efficacy in biomaterials9,18,19,20. In these models, a single implant is typically placed into the tibia, femur, or spinal process per animal, along with bacterial inoculation. As the abscess is developed around or adjacent to the implant following the inoculation, closely mimicking the clinical features, these models are helpful for in vivo study of implant-related infection. However, the outcomes from the quantitative assessments may be inconsistent due to a single implant per subject, the inoculation into an imperfectly enclosed space, and variability in immune responses and infection conditions among subjects. Additionally, the quantitative assessments in these models frequently require specialized expertise and specific measuring apparatus, limiting their applicability. Although the implant size is limited in this mouse model, these issues can be improved by the attributes of this comprehensive methodology, which involves the simultaneous incubation of multiple implants with bacteria under identical infection conditions within a single mouse.
The most critical step in this approach is the removal of the formed biofilm from the implant. A representative method for quantitative evaluation of biofilm is CFU counting, which requires the detachment of bacteria from the biofilm, their suspension in a medium, and subsequent plating11. While mechanical stress techniques involving sonication and vortexing are commonly used to detach biofilm in many studies, there are concerns about their sufficiency in completely removing all biofilm from implants. If these techniques prove to be insufficient, the reliability of the results may be compromised. This study demonstrated that mechanical stimulation alone could not detach the biofilm completely. Therefore, integrating chemical treatment with trypsin and mechanical stimulation is necessary to achieve more reliable bacterial detachment and accurately quantify the live bacterial load within the biofilm21.
The qPCR analysis is commonly used in microbiome studies to assess the overall bacterial load within the biofilm. This assay is likely a more accurate method for quantitative evaluation than the crystal violet assay22. By creating a calibration curve with plasmid DNA purified directly from pure bacterial cultures, the bacterial load within the biofilm can be accurate. Given that the 16S rRNA gene is a ribosomal subunit present in all bacteria, qPCR targeting the 16S rRNA gene is widely utilized for bacterial quantification, and the expression of the 16S rRNA gene is relatively stable during the growth of Staphylococcus aureus23,24. The luxA gene is a component of the lux operon in a bioluminescent strain of Staphylococcus aureus Xen 36. This study demonstrated that qPCR for the luxA gene is also effective for quantitatively evaluating biofilm formation after the inoculation of Xen 36. Thus, the quantitative assessment of biofilm formation can be optimized by inoculating a bioluminescent bacterium and using gene expression analysis of the lux operon. This procedure is relatively easy to implement, reproducible, and enables researchers to analyze multiple samples simultaneously. Moreover, it is economical and requires no special equipment, making it a feasible choice for any laboratory.
This comprehensive approach has some limitations. First, this study primarily aims to assess biofilm formation on implants in a localized and enclosed environment, thereby rendering it insufficient to evaluate the impact of biomaterials on systemic infections. Second, the bacterial load required to form a well-developed abscess in this mouse model is considerably higher than in alternative models, which may create a challenging environment for assessing the resistance of biomaterials to biofilm25. However, to form an abscess completely surrounding the implants and culturing implants under identical bacterial infection conditions, a higher dose of bacteria culture was required. Third, this model cannot be used if one of the implants releases compounds, as they may affect the other implant. Fourth, the pipette tip used to connect the two implants may influence the course of infection, although using a commonly available pipette tip makes the model easy to implement in any laboratory setting.
In conclusion, this study presents a novel mouse model of implant-related infection and optimized analytical methods for precise quantitative assessment of biofilm formation. This comprehensive approach is expected to enhance the accuracy, reproducibility, and versatility of outcome measures for comparative biofilm quantification on surgical implants by exploiting its attributes, thereby contributing to the future development of antimicrobial implants.
Disclosures
The authors declare no competing interests.
Acknowledgements
This research was partially funded by an NSF Industry/University Cooperative Research Program called the Center for Disruptive Musculoskeletal Innovations (IIP-1916629), Komatsuseiki Kosakusho Co., Ltd., and Rosies Base, LLC.
Materials
| Name | Company | Catalog Number | Comments |
| Acetic Acid | THOMAS SCIENTIFIC | 12-16-15-00 | |
| Agilent BioTek 800 TS Absorbance Reader | Agilent | BT800TS | |
| Air-Tite Sterile Hypodermic Needles, Needle Gauge: 18 G | FISHER SCIENTIFIC | 14817151 (CS) | |
| BD Spinal Needles: 25 G | FISHER SCIENTIFIC | 22043805 | |
| Benchtop Incubator Shaker | FISHER SCIENTIFIC | ||
| Branson Ultrasonic Bath, 115 Vac, 60 Hz | FISHER SCIENTIFIC | 2489500 | |
| Branson Ultrasonics 2510R-MTH (Sonicator) | RPI-T48500-500.0 | ||
| Centrifuge sorvall pico | FISHER SCIENTIFIC | ||
| CFX96 Real Time Optics Module qPCR System | BIO-RAD | ||
| Compact Sterilizer, 30L | THOMAS SCIENTIFIC LLC | 22A00N096 (EA/1) | |
| Crystal Violet, 1%, Solution SCI_ED | FISHER SCIENTIFIC | 10114-58-6 | |
| Falcon 24-well cell culture plate | FISHER SCIENTIFIC | 877125 | |
| Falcon 96-well cell culture plate | FISHER SCIENTIFIC | 8771001 | |
| Fisherbrand Digital Vortex Mixer | FISHER SCIENTIFICS | ||
| Kanamycin | THOMAS SCIENTIFIC LLC | 15160054 | |
| Mannitol Salt Agar | FISHER SCIENTIFIC | ||
| Micirobiological Incubator | Thermo Scientific | 51028063H | |
| PBS, Phosphate Buffered Saline | |||
| Staphylococcus aureus ATCC 49525 (Xen36) | Perkin Elmer | 119243 | |
| SYBR Green I Nucleic Acid Gel Stain | FISHER SCIENTIFIC | ||
| Trypsin 10x (2.5%) | FISHER SCIENTIFIC | 15090046 | |
| Tryptic Soy Broth | NETA SCIENTIFIC INC | ||
| Zyppy Plasmid Miniprep Ki | FISHER SCIENTIFIC | 501977785 | pellet-free modified alkaline lysis system |
References
- Wukich, D. K., Lowery, N. J., McMillen, R. L., Frykberg, R. G. Postoperative infection rates in foot and ankle surgery: a comparison of patients with and without diabetes mellitus. J Bone Joint Surg Am. 92 (2), 287-295 (2010).
- Darouiche, R. O. Treatment of infections associated with surgical implants. N Engl J Med. 350 (14), 1422-1429 (2004).
- Arciola, C. R., Campoccia, D., Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat Rev Microbiol. 16 (7), 397-409 (2018).
- Li, P., Yin, R., Cheng, J., Lin, J. Bacterial biofilm formation on biomaterials and approaches to its treatment and prevention. Int J Mol Sci. 24 (14), ijms241411680(2023).
- Chen, X., Zhou, J., Qian, Y., Zhao, L. Antibacterial coatings on orthopedic implants. Mater Today Bio. 19, 100586(2023).
- Pan, C., Zhou, Z., Yu, X. Coatings as the useful drug delivery system for the prevention of implant-related infections. J Orthop Surg Res. 13 (1), 220(2018).
- Savvidou, O. D., et al. Efficacy of antimicrobial coated orthopedic implants on the prevention of periprosthetic infections: A systematic review and meta-analysis. J Bone Jt Infect. 5 (4), 212-222 (2020).
- Sussman, E. M., Casey, B. J., Dutta, D., Dair, B. J. Different cytotoxicity responses to antimicrobial nanosilver coatings when comparing extract-based and direct-contact assays. J Appl Toxicol. 35 (6), 631-639 (2015).
- Cyphert, E. L., Zhang, N., Learn, G. D., Hernandez, C. J., von Recum, H. A. Recent advances in the evaluation of antimicrobial materials for resolution of orthopedic implant-associated infections in vivo. ACS Infect Dis. 7 (12), 3125-3160 (2021).
- Stavrakis, A. I., et al. Current animal models of postoperative spine infection and potential future advances. Front Med (Lausanne). 2, 34(2015).
- Carli, A. V., et al. Quantification of Peri-implant bacterial load and in vivo biofilm formation in an innovative, clinically representative mouse model of periprosthetic joint infection. J Bone Joint Surg Am. 99 (6), e25(2017).
- Bernthal, N. M., et al. A mouse model of post-arthroplasty Staphylococcus aureus joint infection to evaluate in vivo the efficacy of antimicrobial implant coatings. PLoS One. 5 (9), e12580(2010).
- Kelley, B. V., et al. In vivo mouse model of spinal implant infection. J Vis Exp. (160), e60560(2020).
- Meroni, G., et al. A Journey into animal models of human osteomyelitis: A review. Microorganisms. 10 (6), 10061135(2022).
- Wang, Y., et al. Mouse model of hematogenous implant-related Staphylococcus aureus biofilm infection reveals therapeutic targets. Proc Natl Acad Sci U S A. 114 (26), E5094-E5102 (2017).
- Mitsuhiro Nishizawa, D. H., et al. A novel approach to reducing spinal implant-associated infections. bioRxiv. , (2024).
- Fehlings, M. G., et al. A clinical practice guideline for the management of patients with acute spinal cord injury: Recommendations on the use of methylprednisolone sodium succinate. Global Spine J. 7 (3 Suppl), 203S-211S (2017).
- Dworsky, E. M., et al. Novel in vivo mouse model of implant-related spine infection. J Orthop Res. 35 (1), 193-199 (2017).
- Masters, E. A., et al. Distinct vasculotropic versus osteotropic features of S. agalactiae versus S. aureus implant-associated bone infection in mice. J Orthop Res. 39 (2), 389-401 (2021).
- Sheppard, W. L., et al. Novel in vivo mouse model of shoulder implant infection. J Shoulder Elbow Surg. 29 (7), 1412-1424 (2020).
- Mansouri, M. D., Ramanathan, V., Al-Sharif, A. H., Darouiche, R. O. Efficacy of trypsin in enhancing assessment of bacterial colonization of vascular catheters. J Hosp Infect. 76 (4), 328-331 (2010).
- Wang, X., Howe, S., Deng, F., Zhao, J. Current applications of absolute bacterial quantification in microbiome studies and decision-making regarding different biological questions. Microorganisms. 9 (9), 9091797(2021).
- Lima, A., Franca, A., Muzny, C. A., Taylor, C. M., Cerca, N. DNA extraction leads to bias in bacterial quantification by qPCR. Appl Microbiol Biotechnol. 106 (24), 7993-8006 (2022).
- Eleaume, H., Jabbouri, S. Comparison of two standardization methods in real-time quantitative RT-PCR to follow Staphylococcus aureus genes expression during in vitro growth. J Microbiol Methods. 59 (3), 363-370 (2004).
- Vidlak, D., Kielian, T. Infectious dose dictates the host response during Staphylococcus aureus orthopedic-implant biofilm infection. Infect Immun. 84 (7), 1957-1965 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved