Method Article
Development of a Preclinical Inhalation Model to Test Vaporized Cannabis Distillates
In This Article
Summary
Cannabis distillate vape cartridges are battery-powered devices that aerosolize extracts containing high concentrations of cannabinoids. The absence of established preclinical models for these products creates challenges in studying their physiological effects. To address this gap, a standardized preclinical inhalation murine model for vaporized cannabis distillates has been developed.
Abstract
Despite their growing popularity, cannabis vape products remain understudied. Cannabis vape cartridges are used with battery-powered devices that aerosolize cannabis flower extracts containing high concentrations of cannabinoids such as THC. These types of products are commonly known as cannabis distillates. The potency of these products presents challenges in establishing effective dosing for preclinical studies. Currently, there are no established, standardized preclinical models for testing the safety and efficacy of these products in ways analogous to human use patterns. Thus, the in vivo cannabis distillate exposure regime required to achieve physiologically relevant doses in comparison to what is achieved in humans remains undetermined. To address this gap, a standardized preclinical murine model for inhalation of vaporized cannabis distillates has been developed using a computer-controlled delivery system. This protocol details procedures to administer cannabis vape distillates using a regimented puff topography to mice by a nose-only exposure tower. Methods to monitor mouse behavioral outcomes post-exposure and the utilization of a semi-quantitative ELISA to confirm THC delivery into the systemic circulation are also provided. This protocol will allow for the investigation of the pulmonary and systemic responses to cannabis vape distillate products by researchers interested in exploring the impact of cannabis vaping using real-world delivery protocols, thereby providing an opportunity for rigorous safety and therapeutic evaluation.
Introduction
With the legalization of cannabis occurring throughout the world, cannabis use is increasing. Significant changes in the retail cannabis market are not only boosting accessibility, but these changes are also driving the development and production of new types of cannabis products for consumption1. Vaporizers, which heat cannabis products without combustion, are becoming an increasingly popular consumption method2,3. Vaporizers include cannabis vape cartridges that utilize e-cigarette technology to heat and aerosolize cannabis distillates. These distillates are extracted from the Cannabis sativa flower to produce a viscous liquid with high concentrations of cannabinoids such as Δ9-tetrahydrocannabinol (THC), the primary psychoactive component of cannabis4. These devices are easy to use and conceal, making them attractive to novice users5. In Canada, where cannabis was legalized for recreational purposes in 2018, survey data obtained shows an increase in the perceived social acceptability of vaping cannabis as well as significant increases in cannabis vape pen/cartridge usage6.
Cannabis consumers may believe that vaping cannabis distillates is safer than smoking the dried flower in the form of a joint, contributing to its rising popularity2. Despite the possible reduction in exposure to inhaled combustion products when using cannabis vape distillates, these products may not be risk-free. One concern is the exposure to high doses of cannabinoids that are present in commercial vape cartridges. The dried cannabis flower can be purchased with up to 36% THC, whereas the THC concentration in cannabis distillate cartridges can reach as high as 96%7. Aerosols from cannabis distillate cartridges contain approximately twice the THC concentrations compared to cannabis smoke8,9. It is not yet known what effects these elevated THC concentrations have on the respiratory tract. Furthermore, the high concentrations of THC provide challenges in establishing effective dosing for preclinical murine studies, as excessive THC exposure itself may have adverse effects on mice10. It is essential to begin with minimal exposure levels and gradually increase until physiologically relevant doses are achieved to ensure that these exposures are relevant.
To date, there are no studies examining the potential effects of inhaled vaporized cannabis distillates. This is due, in part, to the lack of existing standardized preclinical models. Research challenges are compounded in regions where these products remain illegal, prompting researchers to produce in-house distillates that may not accurately reflect commercial products11. Additionally, the wide array of available products complicates standardization. To bridge this gap, this study was initiated using legal, commercially available products accessible to Canadian consumers. Products and devices listed as top sellers on the Ontario Cannabis Store were selected for use. The goal of this protocol is to establish an easy-to-use murine exposure regimen that delivers THC doses comparable to physiologically relevant human levels, creating a foundation for researchers to conduct additional studies on the respiratory and systemic effects of vaporized cannabis distillates.
Protocol
The procedures below were approved by the McGill University Institutional Animal Care Committee (Protocol Number 8087) in accordance with the guidelines of the Canadian Council on Animal Care (CCAC).
1. Equipment preparation
NOTE: The following protocol applies to the SCIREQ inExpose system supported by the flexiWare 8 software.
- Ensure the correct assembly of all components of the system to resemble the example shown in Figure 1A. Ensure there is a closed tube inserted into the buffer chamber of the puffing pump to prevent system leakage, as shown in Figure 1B.
- Turn on the system and start the software. Select the Experimentation Sessions module.
- Select New Study and define the experimental groups and subjects to be studied. Select the experimental template that corresponds to the desired exposure regimen.
- In the Session Properties window, complete the Operator section to track equipment usage.
- To perform calibration, select the Desired Channel and follow the steps described in the operating software.
- (Critical step) To perform the system flow test, select the Desired Pump and follow the steps described in the operating software. Verify that the system flow is directed toward the rotameter. This calibration is to ensure there is no leakage in the system. If the system does not pass the flow test, clean the components and tubing before re-attempting the test.
- Cancel prompts to start data recording unless ready to start experiment.
2. Creation of Puff profiles
- Create a bias flow profile of 2 L/min to ensure adequate aeration of subjects throughout the experiment. To do so, refer to Technote 037: A Guide to Creating Profiles in an external document available from the manufacturer.
- Create a puff for e-cigarette profiles. To do so, refer to Technote 037. In the protocol described here, the technote was modified to deliver a 78 mL puff volume, with a 2.4 s puff duration, and 1, 2, or 4 puffs/min as previously published12,13,14,15,16,17.
3. Animal preparation
- Use both male and female C57BL/6 mice, aged 10-12 weeks and weighing approximately 20-30 g for this protocol. Acclimate mice to the system gradually over the course of 3 days.
- Begin acclimation with the placement of the mice within the soft restraint, then start bias flow at 2 L/min with room air over a period that would match the length of their experimental exposure. Use an acclimation period of 10 min, 20 min, or 30 min to match the experimental exposures described here. These steps were taken to minimize any stress-induced impacts on outcomes of interest.
- To place animals in soft restraints, fully retract the mesh component, hold the entire restraint in front of the animal, and wait for the animal to move into the plunger component.
- Verify that the animal's nose is visible in the plunger component of the restraint, then secure a binder clip just behind the animal to prevent any movement out of the restraint.
NOTE: Younger, smaller animals can maneuver more easily within the restraint, so it is essential to verify that their nose remains visible outside the plunger component.
- When working with mice of different sexes or genotypes, consider using color-coded binder clips to distinguish between animals.
4. Exposure of animals
- Place six restrained animals in the nose-only tower of the exposure system and initiate the bias flow at 2 L/min with room air to ensure sufficient airflow.
- In the task docker on the right-hand side of the screen, right-click on the E-cigarette Profile Created, then select Task Properties.
- Under Puff Frequency, input the desired frequency for initiating a puff. For example, a 2 puff/min regime requires an input of 30 s. Click OK, then Yes when the software asks for confirmation of this change. To save this puff regime for future use, at the end of the session follow the prompts to save it as a template.
- When ready to perform the exposure, double-click on the created and now modified E-Cigarette Profile. Be sure to start a timer to track the length of the exposure.
- To evaluate the dose over increasing exposure lengths, perform 10 min, 20 min, and 30 min exposures at 1 puff/min. Then, to evaluate increasing exposure intensity, maintain a 10 min exposure length and increase puff frequency to 1 puffs/min, 2 puffs/min, and 4 puffs/min.
- After the exposure is completed, re-initiate the bias flow while returning the animals to their cages.
- To remove animals from the restraints, detach the binder clip and retract the mesh fully to the plunger, allowing the animal to exit the restraint on its own. If the animal remains in the restraint, gently tug its tail to signal that it is free to move backward out of the restraint.
5. Hypo-locomotion test
- Immediately following the exposure, transfer the animals to a behavioral test suite to perform an open field test using the ANY-Maze software. To perform baseline recordings of animal behavior, ensure they are exposed only to bias flow.
- Ensure the room is well-lit and minimize noise levels to prevent unnecessary stress on the animals, as this could influence their behavior.
- Open the software and select New Empty Experiment. Under the Protocol tab at the top of the screen, select Add item, then click New Video Source from the dropdown list to add the camera source to be used for the experiment.
- On the menu on the left side of the screen, under the Tracking section, select Apparatus to define the open field in which the animal will be placed.
- Under the Protocol tab, select the Rectangle Tool to draw the open field area and input its dimensions into the software.
- On the menu on the left side of the screen, under the Tracking section, select Animal Color to specify whether the animal is lighter or darker than the background. This information assists the software to accurately track the animal's movement.
- In the left-hand menu, under the Testing section, select Stages to specify the test duration for the experiment. Input a 60 s test duration.
- Under the Experiment tab, define the experimental treatment and the number of animals per treatment.
- Now, to perform the test, click on the Tests tab. Place the animal into the open field, and click the Green Play Button above the recording of the open field to start the test.
- Upon completion of testing, in the left-hand menu, under the Analysis and Results section, select Results, Reports, and Data and select Total Distance traveled to include this information in the report.
- To generate the report, click the Results tab, and export the data in the desired format.
6. Collection of serum samples
- At 30 min after the exposure, anesthetize animals with an intraperitoneal injection of 250 mg/kg Avertin (2,2,2-tribromoethanol). To ensure proper anesthetization, stimulate the pedal withdrawal reflex by pinching the skin between the toes using blunt forceps. Once the reflex has disappeared completely, the animal is anesthetized deeply enough to be euthanized by exsanguination by cardiac puncture. The 30 min time point is selected to achieve peak serum THC-COOH concentrations post-exposure18.
- Collect blood by cardiac puncture in blood collection tubes, then centrifuge at 10,000 x g for 10 min to separate serum.
- Use serum samples to perform a THC Forensic ELISA to quantify THC-COOH levels as per manufacturer's instructions and as previously published19.
Results
The initial objective was to determine an exposure regime that could deliver physiologically relevant levels of THC to the blood in comparison to humans. Thus, a key component to selecting exposure parameters was to use features that mimicked human use patterns20,21. Male and female C57BL/6 mice were exposed to a Pineapple Express Pax Era Pod containing ~85% THC for 10 min, 20 min, and 30 min at 1 puff/min with a 78 mL puff volume and 2.4 s puff duration. Mice were sacrificed 30 min after the exposure to measure THC-COOH levels. THC-COOH is a secondary metabolite of THC22. In mice, serum THC peaks immediately following exposure and is subsequently hydroxylated by CYP450 enzymes to form 11-OH-THC, which is rapidly oxidized to THC-COOH23,24. THC-COOH then accumulates in the serum, reaching peak concentrations approximately 30 min after exposure, providing a reliable marker for detection18. Serum THC-COOH concentrations were below the limit of detection in air-exposed mice but increased significantly to approximately 11.2 ng/mL, 15.2 ng/mL, and 16.1 ng/mL in mice exposed to the THC vape product for 10 min, 20 min, and 30 min, respectively (Figure 2A). Then, the intensity of the exposures was varied by increasing the number of puffs delivered per minute while keeping all other parameters the same during a 10 min exposure time. Here, serum THC-COOH significantly increased to approximately 11.4 ng/mL, 21.8 ng/mL, and 25.2 ng/mL in mice exposed to THC vape product at 1, 2, and 4 puffs/min, respectively (Figure 2B). The levels of THC-COOH achieved in the 10 min, 2 puffs/min, and 4 puffs/min exposure regimes approximate the levels of THC-COOH found in the serum of human users of cannabis post-inhalation22,25,26,27,28.
After identifying an exposure regime that delivered physiologically relevant cannabinoid doses to mice, the next step was to confirm that this regime would also elicit significant behavioral outcomes. To assess this, a hypo-locomotion test was performed, as it is a component of the tetrad assay commonly used to evaluate behavioral responses to THC in rodents29. Baseline measurements in mice exposed only to bias flow air resulted in a displacement of 3.41 m. After exposure to 10 min of THC vape product at 2 puffs/min, displacement was significantly reduced to 0.29 m, and at 4 puffs/min, it was further reduced to 0.05 m (Figure 3). There was no significant difference in the displacement of the mice between the 2 puffs/min and 4 puffs/min exposures. Thus, the 2 puffs/min exposure regime for 10 min has been selected for use in future studies because it trends towards a smoother recovery for the mice post-exposure while continuing to deliver physiologically relevant doses.
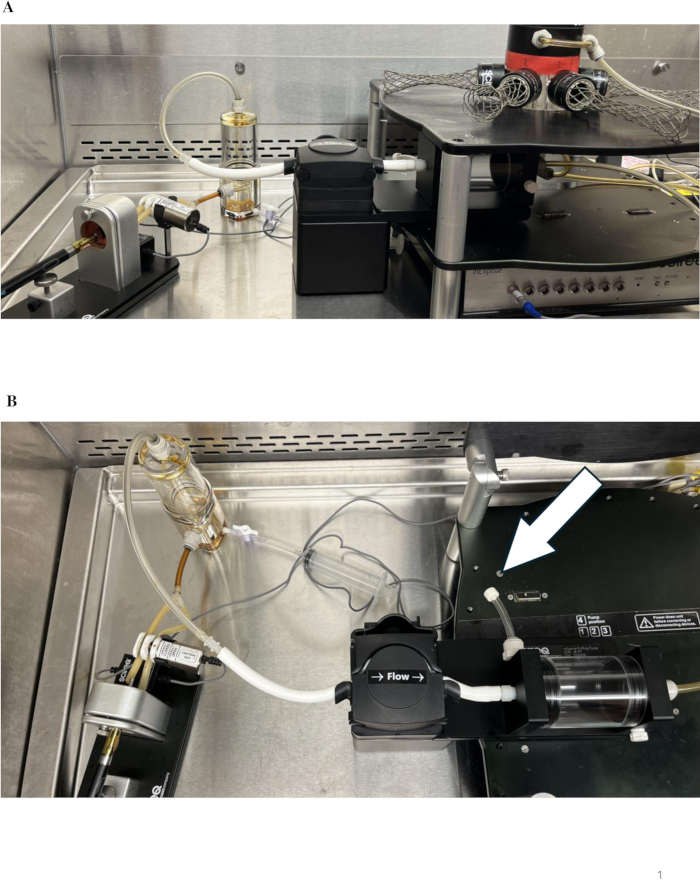
Figure 1: Depiction of the system. (A) Ensure the equipment is assembled correctly, including the appropriate mouthpiece for the specific device, clean tubing, and accurate system flow direction toward the nose-only exposure tower. (B) Ensure the buffer chamber contains a closed tube to prevent leakage, as designated by the arrow in the overhead view. Please click here to view a larger version of this figure.
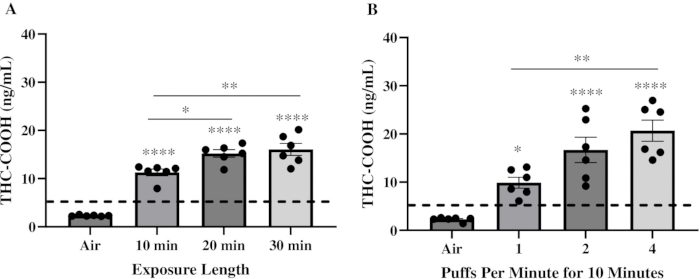
Figure 2: Serum THC-COOH levels after exposure to THC vape product. A total of six mice were exposed to a THC vape product containing 85% THC. At 30 min after exposure, serum was collected to quantify THC-COOH levels by THC ELISA. Graphs represent THC-COOH levels after a (A) 10-, 20- or 30-min exposure at 1 puff/min or (B) after a 10 min exposure at 1, 2 or 4 puffs/min. Results are expressed as mean ± SEM. Data points represent individual mice. The dotted line represents the limit of detection. A one-way analysis of variance (ANOVA) was used to determine significance, with differences between groups examined using Tukey's multiple comparison tests (*p = 0.0348; **p = 0.0022; ****p < 0.0001 compared to air-exposed mice). Please click here to view a larger version of this figure.

Figure 3: Hypo-locomotion test after exposure to THC vape product. A total of six mice were exposed to bias flow air for 10 min then transferred to an open field to conduct a hypo-locomotion test. Upon completion of baseline measurements, mice were exposed to a 10 min exposure at 2 puffs/min or 4 puffs/min. A second hypo-locomotion test was conducted immediately after the exposure. The test duration was 60 s. Results are expressed as mean ± SEM. Data points represent individual mice. A one-way analysis of variance (ANOVA) was used to determine significance, with differences between groups examined using Tukey's multiple comparison tests (****p < 0.0001). Please click here to view a larger version of this figure.
Discussion
Cannabis distillate vape products contain high concentrations of cannabinoids, including up to 85% THC for the product utilized in this protocol. As the cannabis flower currently reaches only as high as 36% THC7, the potency of cannabis distillate vape products provides challenges when developing a model for inhalation exposure. The objective was to determine an exposure regime that could effectively deliver relevant doses of cannabinoids to mice without causing adverse effects from excessive THC exposure levels.
The wide range of cannabis products available for purchase complicates comparisons between studies; however, selecting products with a similar THC potency promotes reproducibility. While the puff parameters are adaptable to various products, the use of lower potency products may require prolonged exposures to ensure reproducibility, coupled with the verification of the delivered systemic dose. Additionally, it is important to note that this study exposed six mice simultaneously. For instance, a 10 min exposure at 2 puffs/min is distributed across six mice, meaning that altering the number of mice per exposure session could affect the individual dose received. Transparent reporting of the number of animals per exposure is essential in future studies using similar equipment, as it could significantly impact the effective dose received by each animal and, consequently, the overall results of the study. Additionally, despite efforts to develop puff profiles that replicate human usage patterns, it is important to note that the exposed mice do not fully inhale each puff that is produced. A considerable portion of the aerosol is released into the surrounding environment. Therefore, verifying the dose delivered into the systemic circulation following exposure is critical to the final interpretation of the data. Furthermore, aerosol monitoring techniques, such as incorporating specialized polytetrafluoroethylene (PFTE) filters within the exposure system to capture aerosol particles, can be utilized to assess particle deposition30,31. Following exposure, the THC content deposited on these filters can be extracted and quantified using liquid chromatography-mass spectrometry (LC-MS)32. Additionally, particle size can be measured using laser diffraction33. By integrating data on aerosol concentration, particle size, and murine respiratory parameters such as tidal volume and breathing frequency, theoretical inhaled doses can be estimated with greater accuracy using computational dosimetry models34,35,36.
In inhalation studies, two primary exposure modalities are used: whole-body and nose-only exposures. In whole-body exposures, animals are unrestrained, allowing the entire body surface to come into contact with the test atmosphere37. A disadvantage of this method is the potential for test agents to deposit on the fur, which may be ingested when the animal grooms itself, introducing an unintended route of exposure38. This can be avoided by using a nose-only inhalation tower. However, nose-only exposures also have limitations. Restraining animals can increase stress, and certain restraints can impair thermoregulation39. This approach can also become labor intensive when working with larger groups of animals. In efforts to mitigate these factors and avoid confounding results, it is essential to place control animals in restraints within the exposure tower, receiving only bias airflow.
Proposed here is a standardized exposure dose that achieves physiologically relevant levels of cannabinoids in comparison to humans. This baseline measurement establishes a foundation for researchers to explore the impacts of both lower and higher exposure doses by modifying puff frequency and exposure duration to determine the effective dose desired for specific experimental needs. Future studies aim to compare various commercial products and devices and assess their respiratory effects using the protocol described here. Given that respiratory researchers frequently use C57BL/6 and Balb/c mice, it is also essential to determine whether the findings presented here are applicable to Balb/c mice to guide future research. Additionally, the protocol's proposed dose is suitable for extended studies investigating acute or chronic effects of cannabis distillate vape products, providing an opportunity for rigorous safety evaluation. Given that the route of exposure is inhalation, it is essential to thoroughly understand the effects of these products on the lungs. Investigating how chronic exposures influence lung mechanics, the function of tissue-resident immune cells, and susceptibility to disease can provide an initial understanding of their impact on lung physiology. Additionally, concerns regarding the long-term effects of these products extend beyond the lungs to other organs. For instance, the chronic effects of potent THC vape exposure on the brain remain poorly understood; however, chronic THC exposure has been linked to cognitive impairment, addiction, and structural alterations in the brain40,41,42,43. Furthermore, given the popularity of these products among youth, their potential impact on brain development warrants investigation. The pursuit of these endpoints will be important as the cannabis marketplace continues to grow, and there is a need for research to investigate the safety of new products. This is vital because it contributes to an improved understanding of the effects of cannabis and helps ensure the safety of millions of cannabis consumers worldwide.
Disclosures
The authors declare they have no conflicts of interest related to this work to disclose.
Acknowledgements
This work was supported by the Canadian Institutes for Health Research (CIHR) Project Grant 162273. CJB was supported by the Fonds de Recherche du Québec -Santé (FRQS).
Materials
| Name | Company | Catalog Number | Comments |
| 2,2,2-Tribromoethanol | Sigma-Aldrich | T48402-5G | Avertin |
| inExpose | SCIREQ | sales@scireq.com | www.scireq.com |
| Microtainer Serum Separator Tubes | BD | 365967 | |
| Pax Era Vape Pen | PAX | Purchased from Ontario Cannabis Store | |
| Pineapple Express Pax Pod | Good Supply | Purchased from Ontario Cannabis Store | |
| SoftRestraints | SCIREQ | IX-XN1-SR-AL | www.scireq.com |
| THC Forensic ELISA Kit | Neogen | 131019 |
References
- Spindle, T. R., et al. Acute Effects of Smoked and Vaporized Cannabis in Healthy Adults Who Infrequently Use Cannabis: A Crossover Trial. JAMA Netw Open. 1 (7), e184841-e184841 (2018).
- Morean, M. E., Kong, G., Camenga, D. R., Cavallo, D. A., Krishnan-Sarin, S. High School Students' Use of Electronic Cigarettes to Vaporize Cannabis. Pediatrics. 136 (4), 611-616 (2015).
- Russell, C., Rueda, S., Room, R., Tyndall, M., Fischer, B. Routes of administration for cannabis use - basic prevalence and related health outcomes: A scoping review and synthesis. Int J Drug Policy. 52, 87-96 (2018).
- Lazarjani, M. P., Young, O., Kebede, L., Seyfoddin, A. Processing and extraction methods of medicinal cannabis: a narrative review. J Cannabis Res. 3 (1), 32 (2021).
- Jones, C. B., Hill, M. L., Pardini, D. A., Meier, M. H. Prevalence and correlates of vaping cannabis in a sample of young adults. Psychol Addict Behav. 30 (8), 915-921 (2016).
- . 2023–24 Departmental Plan: Health Canada Available from: https://www.canada.ca/en/health-canada/corporate/transparency/corporate-management-reporting/report-plans-priorities/2023-2024-departmental-plan.html (2023)
- Geweda, M. M., et al. Evaluation of dispensaries' cannabis flowers for accuracy of labeling of cannabinoids content. J Cannabis Res. 6 (1), 11 (2024).
- Meehan-Atrash, J., Rahman, I. Cannabis Vaping: Existing and Emerging Modalities, Chemistry, and Pulmonary Toxicology. Chem Res Toxicol. 34 (10), 2169-2179 (2021).
- Van der Kooy, F., Pomahacova, B., Verpoorte, R. Cannabis smoke condensate I: the effect of different preparation methods on tetrahydrocannabinol levels. Inhal Toxicol. 20 (9), 801-804 (2008).
- Rosenberg, E. C., Patra, P. H., Whalley, B. J. Therapeutic effects of cannabinoids in animal models of seizures, epilepsy, epileptogenesis, and epilepsy-related neuroprotection. Epilepsy Behav. 70 (Pt B), 319-327 (2017).
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division. . The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. , (2017).
- Been, T., et al. Chronic low-level JUUL aerosol exposure causes pulmonary immunologic, transcriptomic, and proteomic changes. Faseb J. 37 (2), e22732 (2023).
- Been, T., et al. Differential impact of JUUL flavors on pulmonary immune modulation and oxidative stress responses in male and female mice. Arch Toxicol. 96 (6), 1783-1798 (2022).
- Caruana, V., et al. Chronic exposure to E-cigarette aerosols potentiates atherosclerosis in a sex-dependent manner. Toxicol Appl Pharmacol. 492, 117095 (2024).
- Paoli, S., Eidelman, D. H., Mann, K. K., Baglole, C. Sex-specific alterations in pulmonary metabolic, xenobiotic and lipid signalling pathways after e-cigarette aerosol exposure during adolescence in mice. BMJ Open Respir Res. 11 (1), e002423 (2024).
- Lamb, T., Muthumalage, T., Meehan-Atrash, J., Rahman, I. Nose-Only Exposure to Cherry- and Tobacco-Flavored E-Cigarettes Induced Lung Inflammation in Mice in a Sex-Dependent Manner. Toxics. 10 (8), 471 (2022).
- Wang, J., et al. Protein thiol oxidation in the rat lung following e-cigarette exposure. Redox Biol. 37, 101758 (2020).
- Gazarov, E. A., et al. Pharmacokinetics of delta-9-tetrahydrocannabinol following acute cannabis smoke exposure in mice; effects of sex, age, and strain. Front Pharmacol. 14, 1227220 (2023).
- Haidar, Z., Traboulsi, H., Eidelman, D. H., Baglole, C. J. Differential inflammatory profile in the lungs of mice exposed to cannabis smoke with varying THC:CBD ratio. Arch Toxicol. 97 (7), 1963-1978 (2023).
- Behar, R. Z., Hua, M., Talbot, P. Puffing topography and nicotine intake of electronic cigarette users. PLoS One. 10 (2), e0117222 (2015).
- Leavens, E. L. S., et al. electronic cigarette use patterns, other tobacco product use, and reasons for use among ever users: Results from a convenience sample. Addict Behav. 95, 178-183 (2019).
- Huestis, M. A. Human cannabinoid pharmacokinetics. Chem Biodivers. 4 (8), 1770-1804 (2007).
- Hložek, T., et al. Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur Neuropsychopharmacol. 27 (12), 1223-1237 (2017).
- Nasrin, S., Watson, C. J. W., Perez-Paramo, Y. X., Lazarus, P. Cannabinoid Metabolites as Inhibitors of Major Hepatic CYP450 Enzymes, with Implications for Cannabis-Drug Interactions. Drug Metab Dispos. 49 (12), 1070-1080 (2021).
- Hartman, R. L., et al. Controlled Cannabis Vaporizer Administration: Blood and Plasma Cannabinoids with and without Alcohol. Clin Chem. 61 (6), 850-869 (2015).
- Newmeyer, M. N., et al. Free and Glucuronide Whole Blood Cannabinoids' Pharmacokinetics after Controlled Smoked, Vaporized, and Oral Cannabis Administration in Frequent and Occasional Cannabis Users: Identification of Recent Cannabis Intake. Clin Chem. 62 (12), 1579-1592 (2016).
- Schwope, D. M., Karschner, E. L., Gorelick, D. A., Huestis, M. A. Identification of recent cannabis use: whole-blood and plasma free and glucuronidated cannabinoid pharmacokinetics following controlled smoked cannabis administration. Clin Chem. 57 (10), 1406-1414 (2011).
- Sharma, P., Murthy, P., Bharath, M. M. Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iran J Psychiatry. 7 (4), 149-156 (2012).
- Metna-Laurent, M., Mondésir, M., Grel, A., Vallée, M., Piazza, P. V. Cannabinoid-Induced Tetrad in Mice. Curr Protoc Neurosci. 80, 9.59.1-9.59.10 (2017).
- Soo, J. C., Monaghan, K., Lee, T., Kashon, M., Harper, M. Air sampling filtration media: Collection efficiency for respirable size-selective sampling. Aerosol Sci Technol. 50 (1), 76-87 (2016).
- Tang, Z. J., et al. Cytotoxicity and toxicoproteomic analyses of human lung epithelial cells exposed to extracts of atmospheric particulate matters on PTFE filters using acetone and water. Ecotoxicol Environ Safety. 191, 110223 (2020).
- Puah, P. Y., et al. Extractable impurities from fluoropolymer-based membrane filters - interference in high-throughput, untargeted analysis. RSC Adv. 9 (55), 31918-31927 (2019).
- Berrada-Gomez, M. P., Bui, B., Bondarenko, H., Ferret, P. J. Particle size distribution in the evaluation of the inhalation toxicity of cosmetic spray products. Reg Toxicol Pharmacol. 139, 105359 (2023).
- Hammer, T., Gao, H., Pan, Z., Wang, J. Relationship between Aerosols Exposure and Lung Deposition Dose. Aerosol Air Quality Res. 20 (5), 1083-1093 (2020).
- Asgharian, B., et al. Computational modeling of nanoscale and microscale particle deposition, retention and dosimetry in the mouse respiratory tract. Inhal Toxicol. 26 (14), 829-842 (2014).
- Chou, L. T., et al. Particle size matters: Discrepancies in the health risks posed by traditional cigarettes and e-cigarettes in mice and humans. J Hazardous Mater Lett. 4, 100088 (2023).
- Cheng, Y. S., et al. Exposing Animals to Oxidant Gases. Proc Am Thorac Society. 7 (4), 264-268 (2010).
- Chen, L. C., Lippmann, M. Inhalation Toxicology Methods: The Generation and Characterization of Exposure Atmospheres and Inhalational Exposures. Curr Protoc Toxicol. 63 (1), 24.24.21-24.24.23 (2015).
- Wong, B. A. Inhalation Exposure Systems: Design, Methods and Operation. Toxicologic Pathol. 35 (1), 3-14 (2007).
- Cousijn, J., et al. Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. Neuroimage. 59 (4), 3845-3851 (2012).
- Filbey, F. M., Schacht, J. P., Myers, U. S., Chavez, R. S., Hutchison, K. E. Marijuana craving in the brain. Proc Natl Acad Sci U S A. 106 (31), 13016-13021 (2009).
- Sneider, J. T., Gruber, S. A., Rogowska, J., Silveri, M. M., Yurgelun-Todd, D. A. A preliminary study of functional brain activation among marijuana users during performance of a virtual water maze task. J Addict. 2013, 461029 (2013).
- Gilman, J. M., et al. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J Neurosci. 34 (16), 5529-5538 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved