Method Article
Assessing Social Dominance in Mouse Models Using the Tube Test
In This Article
Summary
This protocol describes a behavioral assay to evaluate social dominance in rodents using the tube test. Social dominance remains stable over time, and several models of developmental and neurological disorders exhibit robust social dominance abnormalities. Therefore, the tube test serves as a convenient outcome measure for mechanistic studies or preclinical therapeutic screening.
Abstract
Social dominance is altered in neurodevelopmental and neurodegenerative diseases and serves as a useful outcome measure in preclinical studies of these disorders. The tube test is a simple behavioral assay for evaluating social dominance that does not require expensive equipment. In this test, two mice enter opposite ends of a clear plastic tube, and after meeting in the middle, one (the less dominant) must back out. The tube test can be used for both male and female mice and includes several adaptable parameters to suit the investigator's needs. Mice may be tested against multiple unique opponents to provide an index of social dominance. Social dominance in the tube test remains stable over repeated testing and correlates with performance in other social assays. Additionally, the test can be conducted between cage mates to assess within-cage social dominance hierarchies. The tube test is particularly useful in preclinical therapeutic studies, as it enables longitudinal testing before and after experimental interventions. Therefore, it serves as a convenient outcome measure for mechanistic studies and preclinical therapeutic screening.
Introduction
Disruption of social behavior is a feature of many human disorders, including developmental, psychiatric, and neurodegenerative disorders1,2. Mouse models are used to gain insight into the pathogenesis of these disorders and to provide a platform for the preclinical testing of therapeutics. However, many assays for mouse social behavior are time-consuming to perform, require expensive equipment and/or video-tracking software to analyze, or have subjective scoring algorithms. In contrast, the tube test for social dominance is quick and simple to perform and requires no specialized equipment or video tracking. The assay has a binary win/lose outcome, making the interpretation of results straightforward.
The tube test for social dominance was developed by Lindzey and colleagues in an effort toassess social dominance in mice3. Since its development, the tube test has also been established as a way to assess within-cage social dominance hierarchies4. Tube test phenotypes correlate with other measures of social dominance like barbering, reward competition, and urine-making in male mice5. However, there are mixed results of the tube test and its correlation with competition for food and water access and aggression3,6,7. Importantly, tube test phenotypes correlate with other social phenotypes such as three-chamber sociability8,9.
An advantage of the tube test is that its anatomy is well-defined, making it particularly useful in various mouse models, including models of autism spectrum disorders and other diseases characterized by social deficits like frontotemporal dementia8,9,10,11,12,13,14,15,16. The medial prefrontal cortex (mPFC) is a key mediator of mouse tube test behavior6. Wang and colleagues showed that activity in the mPFC drives social dominance in mice6, and more recent data have refined this insight by showing that mediodorsal thalamic input to the prelimbic and anterior cingulate cortices drives social dominance17. Consistent with a key role for the mPFC in tube test social dominance, abnormalities in dendritic arbors, dendritic spines, glutamate receptors, and/or neuronal excitability, the mPFC has been associated with tube test abnormalities in rodent models of autism spectrum disorders15,18, frontotemporal dementia8,19, chronic stress20, and social isolation21.
Another key advantage of the tube test is the ability to test social dominance both before and after therapeutic intervention, as mouse social dominance in the tube test is stable over time, allowing repeated testing both before and after an experimental intervention6,8,22,23. One example of this comes from progranulin heterozygous (Grn+/−) mouse models of frontotemporal dementia caused by progranulin (GRN) mutations, a haploinsufficiency disease. Progranulin heterozygous mice have a social dominance deficit8. This tube test deficit can be reversed by restoring progranulin with either AAV-progranulin gene therapy22, or administration of anti-sortilin antibodies designed to reduce progranulin degradation23. These examples demonstrate the usefulness of the tube test in designing preclinical trials for dementia-related research.
This protocol provides basic methods for running the tube test between non-cagemates to assess differences between experimental groups, and between cagemates to assess within-cage social dominance hierarchies.
Protocol
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham and performed in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Progranulin heterozygous mice were generated and crossed onto a C57BL/6J background as previously described9. The mice used for this study had been backcrossed onto the C57BL/6J background over at least 12 generations. Mice 9–16 months of age were used in the sample data. Males and females were both used in this study. Mice were kept on a 12:12 h light/dark cycle with lights on at 06:00 h, and all testing was conducted during the light phase. The mice were given ad libitum access to food and water throughout all experiments. The details of the reagents and the equipment used are listed in the Table of Materials.
1. Tube test match design
- Plan the tube test matches before testing. Ensure there is no genotype note to keep the experimenter blind to groups (see Supplementary Table 1).
NOTE: We typically test mice against 3 unique opponents of the opposite experimental group8,9,22,23, but some studies report testing against more opponents10,11,13. - Counterbalance the assignment of experimental mice to the left and right sides of the tube to avoid any potential confounding effects of side bias.
- When testing non-cagemates, minimize the movement of cages to avoid stressing mice more than necessary. To minimize cage movement, overlap matches between the same two cages as much as possible.
- When testing cagemates, test each mouse from a cage against every other mouse in a round-robin fashion. If repeating the test over multiple days, the side of the tube the mouse is assigned to enter (i.e., left or right) should be alternated from day to day.
2. Choice of tubes
NOTE: Typically, the tube test is performed with commercially available clear plastic tubing made of PVC (see Table of Materials). It is important to note that this protocol is optimized for mice but can be applied to other rodent models such as rats, voles, and hamsters2.
- Cut the PVC tubing to a length of 30.5 cm. PVC tubing is slightly flexible and is often stored in rolls, so it may need to be straightened prior to use. Mild heating will help straighten curved tubes.
- The inner diameter of the tube is critical for the success of the test. Ensure that the tube is large enough for the mice to move through, but not large enough to allow the mice to cross over each other.
NOTE: The test will not work if the tube is large enough that the mice can pass each other without engaging in a social dominance contest. The following are approximate guidelines for appropriate tube sizes in C57BL/6J mice: (1) 1 inch/2.5 cm inner diameter (ID) for male mice <6 months old, female mice <9 months old; (2) 1.25 inch/3.2 cm ID for male mice 6-9 months old, female mice >9 months old; and (3) 1.5 inch/3.5 cm ID for male mice >9 months old. - If possible, use a tube of the same size for all tests within a group of mice. If mice cross over during the first few matches, run all subsequent matches with the next smallest tube.
3. Testing location
- Perform the tube test on any flat, stable surface or filtered hood under ambient lighting.
NOTE: The test is typically done during the day between 8 am and 5 pm. For instances where mice are tested across multiple days, each mouse should be tested during the same time of day for each test (e.g., between 11 am and 3 pm). To our knowledge, time-of-day effects have not been previously studied. - Perform the tube test in a quiet location with minimal external stimuli. Ideally, the investigator should be the only person in the room.
4. Habituation
- Habituation is an important practice in behavioral assays that allows mice to become familiar with the testing environment, which can lead to more consistent and reliable results24. For the tube test, acclimate the mice to the testing room prior to testing. If testing is not done in the animals' housing room, allow the mice at least 1 h to habituate to the new room before testing.
- (Optional) If desired, the mice may be habituated to the tube prior to testing. To habituate to the tube, place the mouse in the tube without an opponent and allow it to cross through the tube 2–3 times, 2–3 days prior to testing. This is not our standard practice.
5. Standard tube test
NOTE: (Optional) Investigators new to the tube test may wish to practice placing mice in the tube using a separate non-experimental group of mice. Investigators often need to practice placing mice in the tube. An experienced investigator will induce less stress in the mice undergoing the test. More information on placing mice in the tube is given in step 5.3.
- Place all cages containing mice of the same sex to be tested on a cart. Transport cages to the testing area. Clean the tubes and testing area with 2% chlorhexidine followed by 70% ethanol. The tubes are easily cleaned using a 50 mL serological pipette by pushing a paper towel through the tube.
- Place the two cages for the first match on the testing surface. Remove the lids and place them by each cage.
- Locate the two mice to be tested. Hold one mouse in each hand, corresponding to its assigned side of the tube. After finding the first mouse, gently hold its tail and keep it in the cage while searching for the second.
NOTE: Holding the mouse's tail near the center of its length will allow the investigator to gently control the mouse without restraining it too tightly. Hold the mouse so that all four of its paws remain on the cage bedding to avoid unnecessarily stressing the mouse. - Simultaneously remove both mice from each cage and gently place them with their heads at the entrance to the tube.
NOTE: If the mice have been habituated or have prior test experience, they typically enter the tube within a few seconds. If the mice are naïve to the tube, they may be hesitant to enter for the first trial. If needed, the mice can be switched to the next largest tube to encourage tube entry. - Maintain a grip on each mouse's tail to prevent the mice from making contact prematurely. Ensure that the mice are not forced into the tube. However, once they begin to enter the tube, they may be gently nudged to encourage full entry into the tube.
- When both mice have entered the tube, release the tails and step away from the tube (Figure 1A). Both mice should advance into the tube and meet near the center (Figure 1B).
- Observe the match and record the winner and loser. A mouse is considered to have lost the match when two of its paws exit the tube and contact the testing surface (Figure 1C).
NOTE: One mouse will typically push the other from the tube, though some mice will perform an unforced retreat after contacting the opponent mouse. Occasionally, both mice retreat simultaneously, or one mouse retreats immediately after entering the tube without contacting the opponent mouse. If either of these events occurs, place both mice back in the tube and re-initiate the match. The mice will typically perform a more standard match on the second attempt. If neither mouse has retreated within 2 min, abort the match and run it again at the end of the session.
- Observe the match and record the winner and loser. A mouse is considered to have lost the match when two of its paws exit the tube and contact the testing surface (Figure 1C).
- After the match is over, return the mice to their home cages. Clean the tube and the testing surface with 70% ethanol.
- Continue running matches using the design described in step 1. Each round begins immediately after the preceding round. When all matches from one sex have been run, return the cages to the rack/cart and test the mice of the opposite sex using the same procedure.
- Thoroughly clean the testing surface and tubes between running female and male mice. After testing is complete, clean both the tubes and testing area with 2% chlorhexidine.
- For statistical analysis, count the number of wins for each group and analyze using the binomial test to compare the observed versus expected distribution, with the expected distribution set at 50% wins for each group.
- Calculate the winning percentage for each mouse by dividing the number of wins by the number of matches (typically three matches unless a match was canceled due to a missing mouse or other issues). Compare the winning percentage for each group using the Mann-Whitney test.
NOTE: If repeated, these matches produce similar results in the absence of additional experimental manipulations. For example, a previous study observed that 40 of 47 matches produced the same result when run on successive days8.
- Calculate the winning percentage for each mouse by dividing the number of wins by the number of matches (typically three matches unless a match was canceled due to a missing mouse or other issues). Compare the winning percentage for each group using the Mann-Whitney test.
6. Within-cage tube test for assessing social dominance hierarchies
NOTE: Mice form stable social dominance hierarchies that can be revealed by round-robin tube testing of all mice within a cage4,6. Both male and female mice form these hierarchies8. Social dominance hierarchies are best assessed in cages containing at least 5 mice.
- Plan matches in advance, with counterbalancing as described in step 1.
- Run tube test matches as described in step 5. Test one cage at a time and test all mice in a round-robin fashion. Clean the tube and testing area with 70% ethanol between each match.
- Test each cage once per day for at least 5 days to establish the stable rank orders of each mouse.
- For statistical analysis, assess the number of wins per mouse. Rank each mouse by the number of wins.
7. Using the tube test for preclinical therapeutic screening
NOTE: The robustness and stability of social dominance phenotypes in many mouse models, as well as the ease of performing the tube test, make the tube test a useful paradigm for preclinical testing of therapeutic strategies. For this, the tube test can be done using a within-animal design, as the test can be done serially. It is also possible to do a cross-over design with drug, then control or vice-versa. The tube test has been previously used to evaluate progranulin-boosting therapies in Grn+/− mice22,23, which showed that social dominance phenotypes are reversible in this mouse model. For clarity of the discussion below, mice will be described as "control" (wild-type, nontransgenic, etc.) or "model" (knockout, transgenic, etc.), and experimental interventions will be described as either "vehicle" or "treated" (therapeutic intervention). While mice are referred to as "control" and "model" below, it is ideal to use littermates for each of these groups.
- Before an experimental intervention, perform a pre-test to confirm the presence of the expected social dominance phenotype in the model mice to be used for testing. Test the entire pool of control and model mice against each other and use these data to assign mice to treatment groups to ensure the groups are balanced at baseline.
- Different durations of treatment may be needed based on the therapeutic. For example, if the drug is an AAV, allow the mice to recover from surgery and have time for the drug to take effect. If the drug is a small molecule, determine the treatment paradigms prior to the tube test22.
- Assign mice to experimental groups such that vehicle and treated mice of each genotype have baseline social dominance phenotypes of similar degree. If mice are tested multiple times against several opponents, each mouse's winning percentage (described in step 5.9) can be used to create two groups with similar phenotypes.
- For a truly random distribution of mice into groups based on ARRIVE guidelines, use a block randomization protocol based on the winning percentage of each mouse8.
- After groups are assigned, test the mice at multiple time points during the treatment. For details on typically performed comparisons, see the NOTE below:
NOTE: (1) Vehicle control mice vs. vehicle model mice (this is a control comparison to show that vehicle treatment does not obscure the mouse model's phenotype); (2) vehicle control mice vs. treated model mice (this tests the hypothesis that the treated model mice are different from normal controls); (3) vehicle model mice vs. treated model mice (this tests the hypothesis that the treatment alters the mouse model's phenotype); (4) vehicle control mice vs. treated control mice (this is a control comparison to determine the effects of the treatment in mice without a social dominance phenotype). - Typically, the testing will be performed over two days, with approximately 6 matches per day, with comparisons (1-4) mentioned in step 7.4. To avoid over-testing, run the comparisons (1) and (2) on day 1, and comparisons (3) and (4) on day 2. Since the vehicle control mice are tested against both model groups on day 1, the matches of each comparison must be interspersed.
- After the testing is over, return the mice to their home cages. Clean the tube and the testing surface with 70% ethanol.
Results
The tube test has been extensively used in a mouse model of frontotemporal dementia due to progranulin mutations, Grn+/− mice8,9,22,23,25. These mice exhibit low social dominance by 9 months of age (Figure 2A-D)8. The low social dominance phenotype of older Grn+/− mice is stable through repeated testing (Figure 2A)8, making it a robust experimental outcome measure22,25.
Within-cage testing has also been performed in Grn+/− mice to investigate social dominance hierarchies between cagemates (Figure 3A-D)8. Importantly, both male (Figure 3A,B) and female (Figure 3C,D) mice form these hierarchies, and Grn+/− mice also exhibit low social dominance among their cagemates (Figure 3E). Interestingly, Grn−/− mice did not have this abnormality (Figure 3E).
The low social dominance phenotype exhibited by older Grn+/− mice (Figure 2) makes it an attractive outcome measure for progranulin-boosting therapeutic interventions. Pre-AAV-injection, Grn+/− mice exhibit a low social dominance phenotype (Figure 4A). Grn+/− mice injected with a control virus still show a low-dominance phenotype (Figure 4B). Grn+/− mice injected with a virus to boost progranulin levels no longer have the low social dominance phenotype (Figure 4C). When comparing Grn+/− mice injected with control AAV to Grn+/− mice injected with progranulin-boosting AAV, Grn+/− mice injected with control AAV exhibit low social dominance (Figure 4D).

Figure 1: Schematic of the tube test. (A) Experimenters release the mice once they have entered the tube with all four paws. The tube should be small enough that a mouse can't turn around or climb over another mouse. (B) Both mice then move to the middle of the tube, where they meet. (C) The more dominant mouse will remain in the tube, while the less dominant mouse will back out. The mouse is counted as the loser once two paws exit the tube. Please click here to view a larger version of this figure.

Figure 2: Representative results using the tube test to identify a social dominance phenotype. This study was in the Grn+/− mouse model of frontotemporal dementia. Mice were tested 3 rounds against 3 novel opponents, as described in step 1. Different means of presenting the data are shown. (A) Grn+/− mice over the age of 9 months have a low social dominance phenotype that is stable on repeated testing (* = binomial test, p < 0.05; data points represent percent wins of each genotype). (B) Aggregated data from the three rounds of testing plotting the overall percent wins per genotype (*** = binomial test, p = 0.0004). (C) Plot the number of wins by each mouse across all 3 of its matches. Grn+/− mice had a lower number of wins (**** = Mann-Whitney test, p < 0.0001). Each dot is a mouse. (D) Plot of the percentage of mice in each genotype with a given winning percentage. Grn+/− mice were more likely to have a low winning percentage. (**** = Mann-Whitney test, p < 0.0001). 9–16-month-old mice, n = 58 mice per group. Data are adapted with permission from Arrant et al.8. Please click here to view a larger version of this figure.
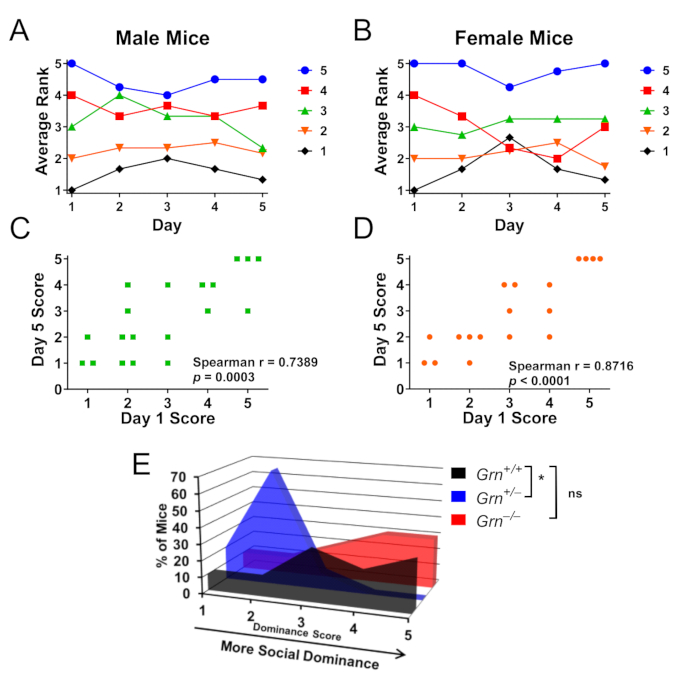
Figure 3: Representative results using the tube test to determine within-cage social dominance hierarchy. Cages of male or female mice (n = 4 cages per sex of 4–5 mice each) were tested in round-robin fashion for 5 days to allow testing of social dominance hierarchy. (A,B) Social dominance scores for male and female mice were determined by number of wins, with 5 being the most dominant mouse and 1 being the least dominant mouse in each cage. At day 5, both male (A) and female (B) mice remained within one rank of their initial score on average. Color coding is by Day 1 ranking. (C, D) Rank on day 5 had a highly significant correlation with the rank on day 1 for both sexes. (E) Using this within-cage paradigm to evaluate cages where Grn+/+, Grn+/−, and Grn−/− mice were group-housed together, Grn+/− mice also exhibited low social dominance (Kruskal-Wallis test, p = 0.0063, * = p < 0.05 by Dunn’s post-hoc test). Interestingly, low social dominance was not observed in Grn−/− mice. Data are adapted with permission from Arrant et al., 20168. Please click here to view a larger version of this figure.

Figure 4: Representative results using the tube test to evaluate preclinical therapeutic efficacy. (A) In baseline testing prior to AAV injection, Grn+/− mice had low social dominance (* = Mann-Whitney test, p = 0.0157; or * = binomial test, p = 0.0281). Using block randomization, mice were then assigned to treatment groups, either an AAV to boost progranulin protein levels (AAV-Grn), or a control AAV (AAV-GFP). (B) Grn+/− mice injected with the control AAV-GFP had low social dominance (* = Mann-Whitney test, p = 0.0196; or * = binomial test, p = 0.0330). (C) Grn+/− mice injected with the AAV-Grn no longer had low social dominance. (D) When comparing Grn+/− mice injected with AAV-GFP vs. AAV-Grn, AAV-Grn injected mice had higher social dominance compared to control AAV-GFP injected mice (** = Mann-Whitney, p = 0.0034; or ** = binomial, p = 0.0062). n = 19-36 mice per group. Data are adapted with permission from Arrant et al.22. Please click here to view a larger version of this figure.
Supplementary Table 1 and 2: Experimental design. An example tube test match design sheet. Note the distribution of each experimental group between the left and right sides of the tube, and the effort to minimize movement of cages in and out of the testing area. Group A is black, and Group B is listed in red to visualize this distribution. Please click here to download this File.
Discussion
The tube test for social dominance provides an easily adopted and quickly performed assay that investigators may find useful as either a primary outcome measure or part of a battery of behavioral tests in mouse models. This article provides basic protocols for performing the tube test between strangers or between cagemates.
Investigators should be aware of several parameters that may affect tube test behavior. For all of these parameters, pilot studies are advisable to determine their effects on a particular mouse model. Both male and female mice may perform the tube test, but mice of each sex should be analyzed separately as some mouse models exhibit sex differences in social dominance phenotype26, while others do not8,15,27,28. The tube test may be repeated multiple times in the same animal, both within a testing session (allowing calculation of a winning percentage for each mouse) and between several sessions (allowing longitudinal testing to assess age or intervention effects). However, repeating the test may affect social dominance phenotypes in some mouse models8,11,26. Finally, background strain has an effect on tube test behavior, as noted in the very first tube test publication3. All of the work described here was performed in mice on the C57BL/6J background, and recent seminal papers on the tube test were also performed in C57BL/6 or C57BL/6J mice4,6,17,29. Investigators working in other strains may wish to confirm the stability and reproducibility of social dominance behavior before proceeding to novel experiments.
The primary outcome measure of the tube test is win/loss. However, other groups have recorded the length of each match and even sub-behaviors within each match. Investigators may, therefore, wish to collect such information to increase the richness of the data obtained from each test. Wang and colleagues reported that matches were shorter when pairing mice with large differences in within-cage hierarchies than when pairing closely ranked mice, showing that the most dominant mice win matches quickly6. Zhou and colleagues scored several sub-behaviors in the tube test: "push-initiated", "push-back", "resistance", and "retreating", and found that winning mice engaged in more pushing and resistance, but less retreating than losing mice17. While video recording of matches is not necessary, investigators interested in these more detailed analyses of social dominance behavior may wish to record each match.
As with many behavioral assays, the tube test may be confounded by other deficits unrelated to social behavior. Motor impairment is likely to impact tube test performance, so investigators may wish to perform a basic screen for motor phenotypes with tests such as the rota-rod, open field, pole test, etc. Olfactory cues are an important aspect of mouse social behavior30,31, so investigators should also screen for olfactory deficits that could impact tube test behavior. A simple way to do this is to measure the time mice spend investigating urine from an unfamiliar mouse versus water9.
Investigators characterizing social behavior in new mouse models should consider using the tube test as part of a battery of social tests. Mouse models with tube test abnormalities often exhibit abnormal social behavior in other assays such as the three-chamber sociability test, resident-intruder test, and qualitative scoring of social interaction9,10,11,12,13,14,15,20,27,32,33,34,35. Social dominance in the tube test correlates with social dominance in other tasks such as barbering, urine marking, and competition for a warm spot in a cage17,36,37. However, social dominance in other tasks, such as competition for food, water, or access to female mice, is less well correlated with tube test social dominance7,38. By using a battery of tests, investigators may obtain measures of social dominance, aggression, social investigation, and social recognition in their mouse models.
The tube test has been used as a primary outcome measure in testing preclinical therapeutic approaches22,23,25 in the Grn+/− mouse model due to the stability of social dominance phenotypes over time and the ability to test mice repeatedly. Investigators interested in using the tube test to screen interventions should first determine the stability of social dominance phenotypes across repeated testing in their mouse model. To do this, first test the mice, then repeatedly test the same matches at intervals of one day, one week, or one month. If longer experimental timelines are needed, these repetitions can be carried out at even longer intervals. The agreement between these matches can be statistically determined by calculating the kappa value between the tests. If the tube test achieves suitable stability in the mouse model, it makes an ideal screening assay due to its simplicity and speed.
Disclosures
Erik D. Roberson has served as a consultant for AGTC and Lilly and received royalties from Genentech.
Acknowledgements
We thank James Black and Miriam Roberson for help with mouse breeding and colony maintenance, Anthony Filiano and Alicia Hall for assistance with tube test pilot assays, and Robert Farese, Jr. for providing progranulin knockout mice. This work was supported by the Consortium for FTD Research and the Bluefield Project to Cure FTD, the National Institute of Neurological Disorders and Stroke (R01NS075487, P30NS047466, and F32NS090678), and the National Institute on Aging (P30AG086401, R00AG056597, and K00AG068428). Behavior experiments were performed in the Animal Behavior Assessment Core Facility at the University of Alabama at Birmingham.
Materials
| Name | Company | Catalog Number | Comments |
| Animals diet | Envigo | NIH-31 diet #7917 | Animals chow |
| CHLORHEXIDINE 2% SOLUTION 1GAL | Patterson Veterinary Supply INC | 78924243 | For cleaning tubes and surface |
| Ethanol 70% | Vion Biosciences | VNEE0069CS/4 | For cleaning tubes and surface |
| Large tube for male mice > 9 months old | Home Depot | Store SKU # 1000017942 | 1-7/8 in. O.D. x 1-1/2 in. I.D. x 24 in. Clear PVC Vinyl Tube |
| Medium tube for male mice 6–9 months old, female mice > 9 months old | Home Depot | Store SKU # 1000017945 | 1-5/8 in. O.D. x 1-1/4 in. I.D. x 24 in. Clear PVC Vinyl Tube |
| Small tube for male mice < 6 months old, female mice < 9 months old | Home Depot | Store SKU # 1000017938 | 1-3/8 in. O.D. x 1 in. I.D. x 24 in. Clear PVC Braided Vinyl Tube |
References
- Choi, T. Y., Jeong, S., Koo, J. W. Mesocorticolimbic circuit mechanisms of social dominance behavior. Exp Mol Med. 56 (9), 1889-1899 (2024).
- Fulenwider, H. D., Caruso, M. A., Ryabinin, A. E. Manifestations of domination: Assessments of social dominance in rodents. Genes Brain Behav. 21 (3), e12731(2022).
- Lindzey, G., Winston, H., Manosevitz, M. Social dominance in inbred mouse strains. Nature. 191, 474-476 (1961).
- Fan, Z., et al. Using the tube test to measure social hierarchy in mice. Nat Protoc. 14 (3), 819-831 (2019).
- Cum, M., et al. A multiparadigm approach to characterize dominance behaviors in CD1 and c57BL6 male mice. eNeuro. 11 (11), (2024).
- Wang, F., et al. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science. 334 (6056), 693-697 (2011).
- Van De Weerd, H. A., Van Den Broek, F. A., Beynen, A. C. Removal of vibrissae in male mice does not influence social dominance. Behav Processes. 27 (3), 205-208 (1992).
- Arrant, A. E., Filiano, A. J., Warmus, B. A., Hall, A. M., Roberson, E. D. Progranulin haploinsufficiency causes biphasic social dominance abnormalities in the tube test. Genes Brain Behav. 15 (6), 588-603 (2016).
- Filiano, A. J., et al. Dissociation of frontotemporal dementia-related deficits and neuroinflammation in progranulin haploinsufficient mice. J. Neurosci. 33 (12), 5352-5361 (2013).
- Shahbazian, M., et al. Mice with truncated mecp2 recapitulate many Rett syndrome features and display hyperacetylation of histone h3. Neuron. 35 (2), 243-254 (2002).
- Spencer, C. M., Alekseyenko, O., Serysheva, E., Yuva-Paylor, L. A., Paylor, R. Altered anxiety-related and social behaviors in the fmr1 knockout mouse model of fragile x syndrome. Genes Brain Behav. 4 (7), 420-430 (2005).
- Irie, F., Badie-Mahdavi, H., Yamaguchi, Y. Autism-like socio-communicative deficits and stereotypies in mice lacking heparan sulfate. Proc Natl Acad Sci U S A. 109 (13), 5052-5056 (2012).
- Veenstra-Vanderweele, J., et al. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci U S A. 109 (14), 5469-5474 (2012).
- Jiang-Xie, L. F., et al. Autism-associated gene dlgap2 mutant mice demonstrate exacerbated aggressive behaviors and orbitofrontal cortex deficits. Mol Autism. 5, 32(2014).
- Huang, W. H., et al. Early adolescent rai1 reactivation reverses transcriptional and social interaction deficits in a mouse model of smith-magenis syndrome. Proc Natl Acad Sci U S A. 115 (42), 10744-10749 (2018).
- Rascovsky, K., et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 134 (Pt 9), 2456-2477 (2011).
- Zhou, T., et al. History of winning remodels thalamo-PFC circuit to reinforce social dominance. Science. 357 (6347), 162-168 (2017).
- Zhou, X., et al. Prosaposin facilitates sortilin-independent lysosomal trafficking of progranulin. J Cell Biol. 210 (6), 991-1002 (2015).
- Cook, A. K., et al. Dendritic spine head diameter is reduced in the prefrontal cortex of progranulin haploinsufficient mice. Mol Brain. 17 (1), 33(2024).
- Park, M. J., Seo, B. A., Lee, B., Shin, H. S., Kang, M. G. Stress-induced changes in social dominance are scaled by AMPA-type glutamate receptor phosphorylation in the medial prefrontal cortex. Sci Rep. 8 (1), 15008(2018).
- Tada, H., et al. Neonatal isolation augments social dominance by altering actin dynamics in the medial prefrontal cortex. Proc Natl Acad Sci U S A. 113 (45), E7097-E7105 (2016).
- Arrant, A. E., Filiano, A. J., Unger, D. E., Young, A. H., Roberson, E. D. Restoring neuronal progranulin reverses deficits in a mouse model of frontotemporal dementia. Brain. 140 (5), 1447-1465 (2017).
- Kurnellas, M., et al. Latozinemab, a novel progranulin-elevating therapy for frontotemporal dementia. J Transl Med. 21 (1), 387(2023).
- Ueno, H., et al. Effects of repetitive gentle handling of male c57bl/6ncrl mice on comparative behavioural test results. Sci Rep. 10 (1), 3509(2020).
- Arrant, A. E., Nicholson, A. M., Zhou, X., Rademakers, R., Roberson, E. D. Partial tmem106b reduction does not correct abnormalities due to progranulin haploinsufficiency. Mol Neurodegener. 13 (1), 32(2018).
- Chachua, T., et al. Sex-specific behavioral traits in the BRD2 mouse model of juvenile myoclonic epilepsy. Genes Brain Behav. 13 (7), 702-712 (2014).
- Lijam, N., et al. Social interaction and sensorimotor gating abnormalities in mice lacking dvl1. Cell. 90 (5), 895-905 (1997).
- Nishimura, I., Yang, Y., Lu, B. Par-1 kinase plays an initiator role in a temporally ordered phosphorylation process that confers tau toxicity in drosophila. Cell. 116 (5), 671-682 (2004).
- Zhang, C., et al. Dynamics of a disinhibitory prefrontal microcircuit in controlling social competition. Neuron. 110 (3), 516-531.e6 (2022).
- Lin, D. Y., Zhang, S. Z., Block, E., Katz, L. C. Encoding social signals in the mouse main olfactory bulb. Nature. 434 (7032), 470-477 (2005).
- Matsuo, T., et al. Genetic dissection of pheromone processing reveals main olfactory system-mediated social behaviors in mice. Proc Natl Acad Sci U S A. 112 (3), E311-E320 (2015).
- Koh, H. Y., Kim, D., Lee, J., Lee, S., Shin, H. S. Deficits in social behavior and sensorimotor gating in mice lacking phospholipase cbeta1. Genes Brain Behav. 7 (1), 120-128 (2008).
- Long, J. M., Laporte, P., Paylor, R., Wynshaw-Boris, A. Expanded characterization of the social interaction abnormalities in mice lacking dvl1. Genes Brain Behav. 3 (1), 51-62 (2004).
- Moretti, P., Bouwknecht, J. A., Teague, R., Paylor, R., Zoghbi, H. Y. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum Mol Genet. 14 (2), 205-220 (2005).
- Nishijima, I., et al. Secretin receptor-deficient mice exhibit impaired synaptic plasticity and social behavior. Hum Mol Genet. 15 (21), 3241-3250 (2006).
- Rodriguiz, R. M., Chu, R., Caron, M. G., Wetsel, W. C. Aberrant responses in social interaction of dopamine transporter knockout mice. Behav Brain Res. 148 (1-2), 185-198 (2004).
- Greenberg, G. D., Howerton, C. L., Trainor, B. C. Fighting in the home cage: Agonistic encounters and effects on neurobiological markers within the social decision-making network of house mice (mus musculus. Neurosci Lett. 566, 151-155 (2014).
- Benton, D., Dalrymple-Alford,, John, C., Brain, P. F. Comparisons of measures of dominance in the laboratory mouse. Anim Behav. 28, 1274-1279 (1980).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved