Method Article
Studying the Coding Profiles of Somatic Stimulation on Cardiac-locked Neuronal Responses in the Rat Spinal Dorsal Horn
In This Article
Summary
We describe a protocol for spinal multichannel extracellular recording alongside cardiac function recording and analyzing the cardiac-locked spinal dorsal horn neurons. This method offers a temporally synchronized framework for studying spinal mechanisms underlying thoracic visceral functional changes induced by acupuncture.
Abstract
Many studies have suggested that electroacupuncture may be beneficial in the treatment and prevention of cardiovascular disease. However, its mechanism remains poorly understood. The thoracic spinal dorsal horn (SDH) plays an important role in integrating and modulating somatic and visceral inputs, which may then influence cardiac control. In contrast to the lumbar SDH, which has been extensively studied, thoracic SDH has been less explored due to the difficulty in surgical exposure and stereotaxic fixation. In this study, we provide a general approach for simultaneously monitoring neuronal activity and cardiac function by combining the recording of electrocardiograms and microelectrode arrays. Furthermore, we describe how to identify cardiac-locked neurons by calculating the firing rate distribution of neuronal activity in sync with heartbeats. The strategy is of great significance for studying the correlation between cardiovascular function and neuronal activity as well as for understanding the somatocardiac reflex triggered by peripheral nerve stimulations.
Introduction
Acupuncture or body surface stimulation, as a prominent therapeutic technique within the framework of Traditional Chinese Medicine (TCM), operates by stimulating specific areas on the body surface. It facilitates multi-level regulation of the organism's functions through the regulation of visceral functions via afferent pathways, central integration, and autonomic efferent nervous mechanisms. Central to this therapy is the concept that targeted stimulation of anatomically defined acupoints induces systemic physiological regulation. Growing clinical evidence supports acupuncture's role as a complementary modality in managing cardiovascular disorders, with demonstrated efficacy in both primary prevention and adjunctive treatment protocols1,2.
Primary afferents of sensory neurons predominantly terminate in the spinal dorsal horn (SDH), correspondingly, the spinal dorsal horn neurons (SDHNs) play a crucial role in the integration and modulation of somatic inputs3,4,5. Furthermore, the SDHRNs also receive cardiac afferents and convey visceral information to spinal sympathetic preganglionic neurons (SPNs) for cardiovascular modulation6. The cardiac-locked SPNs are located at the lateral corner of the thoracic segment of the spinal cord (T1-T5), with axons projecting to the cervical or thoracic ganglia and subsequently innervating the heart via the cardiac, middle, and lower nerves. As a result, the thoracic spinal cord plays a crucial role in the integration and modulation of somatic and visceral inputs, which may then influence cardiac control. It is thus important to understand how somatic stimulation regulates cardiac function through modulation of the SDHRNs in the thoracic segment of the spinal cord.
Previous studies have demonstrated that electroacupuncture at PC6 (organized in the T3 spinal segment as a homotopic structure-function unit) can alleviate symptoms of myocardial ischemia through modulation of the autonomic nervous system7,8,9. However, real-time quantitative synchronization of acupuncture's effects on heart rate with nervous system activity has not yet been realized. Only immediate autonomic nervous activity and electrocardiogram (ECG) indicators following acupuncture have been documented. Research connecting SDHNs with visceral physiological functions remains scarce. Owing to the physiological curvature of the thoracic vertebrae and the narrow space between adjacent thoracic vertebral segments, especially T1-T5, accessing these areas is challenging, resulting in scant direct evidence for elucidating the spinal mechanisms underlying acupuncture at the T3 spinal homotopic acupoint PC6 regulating cardiac function in the treatment of CVD.
To better understand the relationship between SDH and acupuncture-mediated cardiac function regulation, synchronous recording of cardiac function and neural activities needs to be implemented. Here, we will provide a general approach for spinal multichannel extracellular recording alongside cardiac function recording as well as analyzing the cardiac-locked SDHRNs. This method offers a temporally synchronized framework for studying spinal mechanisms underlying thoracic visceral functional changes induced by acupuncture.
Protocol
The animal experiment protocol strictly adhered to the requirements of the national standard "Guidelines for Ethical Review of Welfare of Laboratory Animals" (GB/T 35892-2018) and was approved by the Ethics Committee of the institution. Male SPF-grade Sprague-Dawley (SD) rats, aged 6-8 weeks and weighing approximately 220 g, were used in this study. Laboratory gowns, gloves, and masks were worn during all the experiments. The details of the reagents and the equipment used are listed in the Table of Materials. At the endpoint of the experiment, rats were euthanized via cardiac perfusion under deep anesthesia followed by cervical dislocation.
1. Preoperative setup
- Connect the ventilator circuit to the Y-shaped endotracheal tube, ensuring a secure and airtight interface.
- Verify proper ventilation by confirming stable airflow, appropriate tidal volume, and respiratory rate settings on the ventilator display.
- Ensure the absence of condensate or particulate contaminants within the tubing by visually examining all segments under bright illumination.
- Simultaneously feed the amplified ECG signals into both the electrocardiograph input and the microelectrode array recording system's analog input port using a three-way BNC connector.
- Route the amplified signals through a three-way BNC splitter to simultaneously connect the electrocardiograph analog input and microelectrode array recording system's analog input port.
- Establish synchronization triggers by connecting the electrocardiograph's TTL output to the microelectrode array recording system's digital input. Using a BNC-to-Dupont interface cable, initiate concurrent acquisition on both systems, verifying temporal alignment.
2. Preoperative preparation
- Anesthetization
- Induce anesthesia with 3-5% isoflurane by inhalation and then, administer an intraperitoneal injection of pentobarbital sodium (50 mg/kg) to the anesthetized rat. Administer isoflurane for inhalation anesthesia before placing the electrodes, maintaining a concentration of ~1.2%. Proceed only if Assess the depth of anesthesia via toe pinch before proceeding.
- Remove the hair from the anterior neck and posterior back of the rats.
- Position the rat in a supine position on a warming blanket and apply ointment to the rat's eyes to prevent drying. Observe the respiration rate and check for withdrawal responses by applying pressure to the footpad with forceps.
3. Tracheal intubation
- Place the rats in a supine position and disinfect the neck area with iodine tincture.
- Perform a longitudinal incision of approximately 1 cm along the midline of the neck and bluntly dissect the muscle tissue.
- Once the thyroid gland is exposed, carefully separate the thin membrane between the two thyroid lobes, taking care not to damage the thyroid tissue. Proceed to expose the trachea.
- Examine the "Y-shaped cannula to confirm that it is completely dry. Employ spring scissors to make a transverse incision in the trachea, followed by the insertion of the cannula into the tracheal opening. Secure the tracheal cannula with 3-0 non-absorbable sutures to prevent air leakage and accidental extubation.
- Carefully suture the neck muscles and skin and connect the rat to a ventilator. Adjust the respiratory rate to 85 breaths/min and the tidal volume to 3.5 mL, according to the rat's body weight7 (Figure 1B).
4. ECG detection
- Insert three electrodes into the rat's skin: the positive electrode into the left lower limb, the negative electrode into the right upper limb, and the ground electrode into the right lower limb10.
- Acquire data using an electrocardiograph (filter settings: low-pass at 100 Hz, high-pass at 1 kHz; sampling frequency: 4 kHz/s). Use ECG recording software to record, save, and analyze data.
5. Pericardial catheterization for drug administration bradykinin (BK)
- Place the rat in a supine position and disinfect the skin on the chest with iodine.
- Perform a thoracotomy between the 1st and 3rd costal cartilages on the left upper chest to expose the thymus. Bluntly dissect the thymus along the midline to expose the pericardium (see Figure 1C,D).
- Use the tip of a glass dissecting needle (0.5 mm in diameter) to make a small opening in the pericardium.
- Insert a silicone catheter, 10-15 cm in length, with several small holes at its distal end through the incision in the pericardium. Secure the catheter to the chest wall tissue using bioglue.
- Close the chest cavity layer by layer, ensuring the rat's breathing remains unobstructed (see Figure 1D).
6. Exposure of T3 spinal cord
- Place the rat in a prone position and perform routine disinfection with iodine. Make an incision of approximately 8 cm along the midline of the back from the T2 to T6 vertebrae.
- Use spring scissors to cut through the skin and muscle layers, including the trapezius muscle. Insert a retractor between the muscles to further expose the surgical field.
- Carefully separate the fat and hibernation glands at the anterior aspect of the rat's thoracic vertebrae, avoiding the blood vessels beneath the glands (see Figure 1E).
- Remove the muscles attaching to the head clamp and the straight portion of the long neck muscles, exposing the spinous processes of T2.
- Displace the semispinalis and spinalis muscles to expose the vertebral arch from T2 to T6 (see Figure 1E). Use rongeurs to remove the spinous process of the T3 vertebra, thereby exposing the T3 spinal cord.
NOTE: During the process of exposing the thoracic vertebrae, ensure special attention is given to preserving the spinous process of T2, as it serves as the primary point of force application for subsequent exposure of T3 spinal cord. - Remove the dura mater and arachnoid membrane, and drip paraffin oil onto the surface of the spinal cord to maintain the viability of the spinal neurons (see Figure 1F).
NOTE: The blood vessels of the rat's brown adipose tissue originate at the T3, T4, or T5 lamina and are distributed like a venous sinus. Take care not to touch them, as this could cause excessive blood loss in the rat.
7. Thoracic vertebrae fixation and settings
- Use a custom spinal clamp to secure the articular processes of T2 and T6. Moisten the surrounding muscles with saline to maintain hydration.
- Attach the electrode array to the micromanipulator of a stereotactic instrument and insert it vertically into the dorsal horn of the spinal cord at the T3 spinal segment through the dorsal median sulcus, 500 µm lateral to the midline, to a depth of 1,500 µm.
- Insert the reference electrode into the back muscle (see Figure 1G).
- Launch the multichannel extracellular recording software and navigate to File | Hardware Configuration; select the 32 channel array from the device interface list; right-click on the selected channel group; and then, choose Properties from the context menu. Configure signal processing parameters: Under the Filter tab, set the bandpass filter (BP) to 250 Hz - 5 kHz; in the Sampling Rate field, input 30 kHz/s; enable spike detection algorithms by checking the box labeled Enable Spike Processing to activate real-time spike sorting and threshold-based event extraction.
NOTE: Ensure thorough clearance of muscles and tissues surrounding the articular processes of T2 and T6, especially in the region where the custom spinal clamp is applied for spinal fixation, to prevent displacement during subsequent experiments.
8. Somatic and BK stimuli
- Use BK (1 µg/mL in distilled water) to induce cardiac nociceptive stimulation. Inject 4 µL of the BK solution with a micro-syringe connected to a silicone catheter with several small openings11.
- Observe heart rate changes (increases or decreases) and neuronal discharge (increases or decreases) in the T3 spinal cord dorsal horn within 30 min of the injection to Identify dynamic interactions between thoracic spinal cord dorsal horn neurons.
- Perform manual acupuncture at acupoint PC6 (MAPC6) using the stimulation parameter of 1 Hz. PC6 (Neiguan acupoint) is located 2 mm proximal to the carpal joint on the ventral forearm, between the flexor carpi radialis and the median nerve trunk. Insert the needles (0.25 mm x 25 mm) into the PC6 acupoints at a depth of ~3 mm. Compare the changes in neuronal activity and cardiac function before and after the somatic stimuli.
9. Data analysis and processing
- Import the recorded neural data in ns6 format into the software follows:
- File Conversion: Navigate to File | Open to load the ns6 file. Select File | Save As and choose the .nex5 format to generate standardized spike train data.
- Spike Sorting: Import the converted .nex5 files into the classification softwarefor neuronal classification. Sort spike waveforms based on waveform characteristics and principal component analysis (PCA), with threshold parameters set at ±3 SD from baseline noise.
- Then, execute the relevant code for filtering and categorizing the signals.
- Analyze cardiac-locked SDRNs.
- Taking the R-wave as the reference event, count the number of neuron firings within a 0.2 s window before and after each R-wave.
- After counting the R-waves at 50 ms intervals, create a ring event histogram. Normalize the histogram (i.e., subtract the average discharge rate in 0.2s before and after the R-wave event) to obtain the discharge rate distribution of each neuron's activity during the heartbeat.
- Assess statistical significance via Monte Carlo12 permutation testing, implemented with 1,000 shuffled iterations. Obtain the firing rate distribution and confidence interval of the neuron in the process of the randomized heartbeat by randomizing 0.2 s of the time before and after each heartbeat R wave (the range is ± 0.1 s). If the firing distribution of a neuron's heartbeat exceeds (greater than or less than) the 95% confidence interval of the firing rate distribution of the randomized heartbeat process, identify the neuron as a cardiac-locked neuron (see Supplemental File 1).
Results
Following the above protocol, the T3 SDHNs were exposed, with bradykinin (BK) or somatic needling administered to pericardial/acupoint regions. This investigation quantified stimulus-evoked neuronal activation profiles (type/frequency) and concurrent electrocardiographic (ECG) changes during nociceptive visceral input, BK application, and somatosensory modulation.
Figure 2A shows a transverse slice of the rat's T3 spinal cord. On the left side, it illustrates the distribution of distinct laminae. The right side displays orange regions representing the Dil dye distribution after a 10 min incubation period. An array electrode, impregnated with Dil dye, was affixed to the micromanipulator of a stereotactic apparatus. The electrodes were then vertically inserted into the dorsal horn of the spinal cord, 500 µm lateral to the posterior median sulcus at the T3 segment, to a depth of 1,500 µm, with depth adjustments made according to the rat's body weight. Following a 15 min recording period, perfusion and tissue collection were conducted, during which the electrodes recorded signals from laminae I to VIII of the rat spinal cord. Figure 2B-D presents the raster plots, waveforms, principal component analysis (PCA), and autocorrelation of neurons recorded from channel 19. Figure 2F,G show analogous data for three neurons recorded from channel 11. The data from these two channels indicate a clear classification of neurons, providing authentic data for subsequent correlation analysis.
The blue columns show the cumulative number of neuronal discharges within a certain time frame, subtracted by the average value. Figure 3A shows a baseline (BL) recording for 60 s. Channel 17c and channel 21a neurons demonstrate the presence of cardiac-locked neurons in the T3 SDHNs, exhibiting distinct periodic discharges in response to ECG variations. Figure 3B shows the effect of BK application to the pericardium in the left atrium on T3 SDHNs, along with an example of cardiac-locked neurons. Channel 17c neuron showed a significant reduction in cardiac-locked after BK administration. As depicted in the green shaded area of Figure 3C, following the administration of MAPC6, there is a consistent cluster of neurons that exhibit periodic firing around the R-wave in the firing cycles of both BL and MAPC6 conditions. This pattern is more regular than the firing observed after BK application. In contrast, in Figure 3D, the firing pattern of neurons post MAPC6 is less distinct. Thus, MAPC6 can improve the firing frequency of cardiac-related neurons in the T3 spinal dorsal cornea, thereby maintaining cardiac function under pathological conditions. Figure 3E,F shows that the discharge frequency of T3 SDHNs activated by BK decreased after acupuncture stimulation of the PC6 acupoint. Conversely, the firing frequency proportion of T3 spinal neurons inhibited by BK was significantly increased after acupuncture stimulation of the PC6 acupoint.
Interestingly, channel 21a is identified as a cardiac-locked neuron that exhibits periodic, regular discharges specifically in response to the P wave, which marks the initiation of atrial depolarization by the sinoatrial (SA) node, and the PR interval, which indicates the delay at the atrioventricular node. Although there is no significant change in the P wave in the ECG, the application of BK results in a concentration of three clusters of neurons between each P and Q wave on the ECG, demonstrating a closer relationship with the P wave (as indicated by the purple shaded area in Figure 3B). The P wave on the ECG is an important indicator reflecting the normality of atrial contraction waves, and the P wave generated by the SA node represents the process of atrial depolarization, thereby the effect of BK on the atria was verified. However, following the administration of MAPC6, there was a distinct pattern in the firing of neurons in relation to the R and T waves. After acupuncture, the neurons reverted to a clustered pattern associated with the P wave, indicating that acupuncture can treat pathological heart diseases by modulating the rhythm of somatic and visceral neurons in the same segment of the spinal dorsal horn.

Figure 1: Surgical procedure of the T3 SDH. (A) Ventilator. (B) Intubation of trachea in rat. (C) Thymus and heart anatomy. (D) Pipe thymus into the pericardium. (E) T2-8 Thoracic vertebrae after removing. (F) T3 spinal cord. (G) The modified spinal vertebrae clampers. (H,I) Local fixation of spinal clamp. (J,K) A schematic diagram of somatic and visceral stimulation; during recording, the rats were in supine and prone positions. Please click here to view a larger version of this figure.
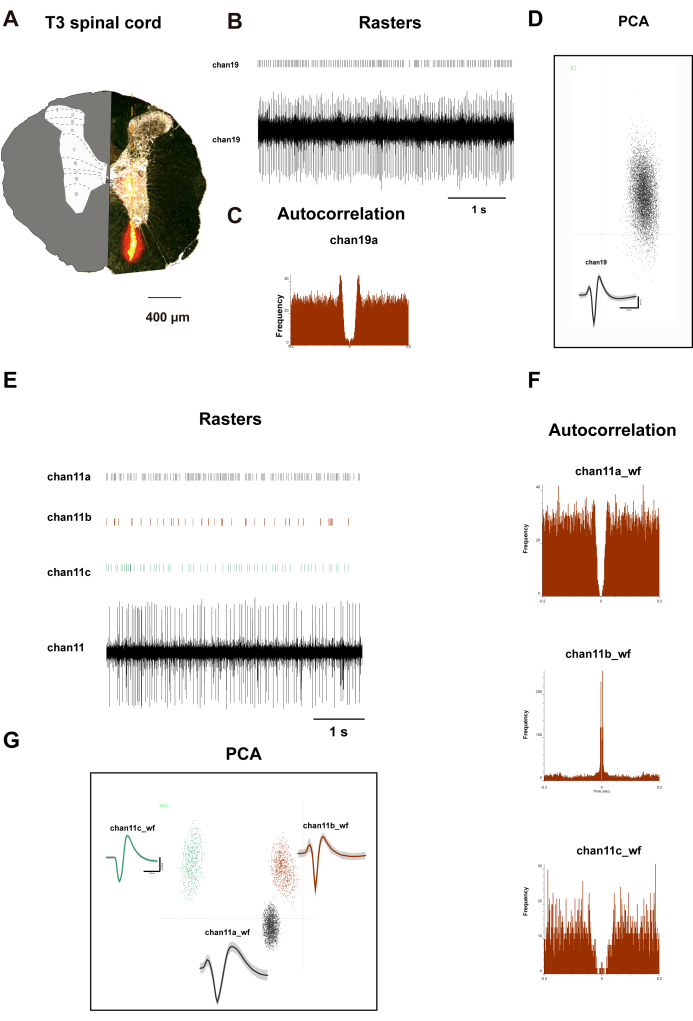
Figure 2: T3 spinal dorsal horn neurons recorded. (A) Electrode placement validation: Transverse spinal cord section showing implantation track of 32-channel silicone probe. Inset: Modified Paxinos rat spinal cord atlas with cytoarchitectonic laminae boundaries (I-X) superimposed for spatial registration. The graphs depict (B,E) channel 19 and channel 11 neuronal firing rasters and waveforms, (D,G) PCA clustering and Cluster diagram, and (C,F) autocorrelations. The centrally symmetrical patterns depicted in panels C and F show the distinctive firing patterns of neurons recorded on channel 19 and channel 11 neurons. Please click here to view a larger version of this figure.
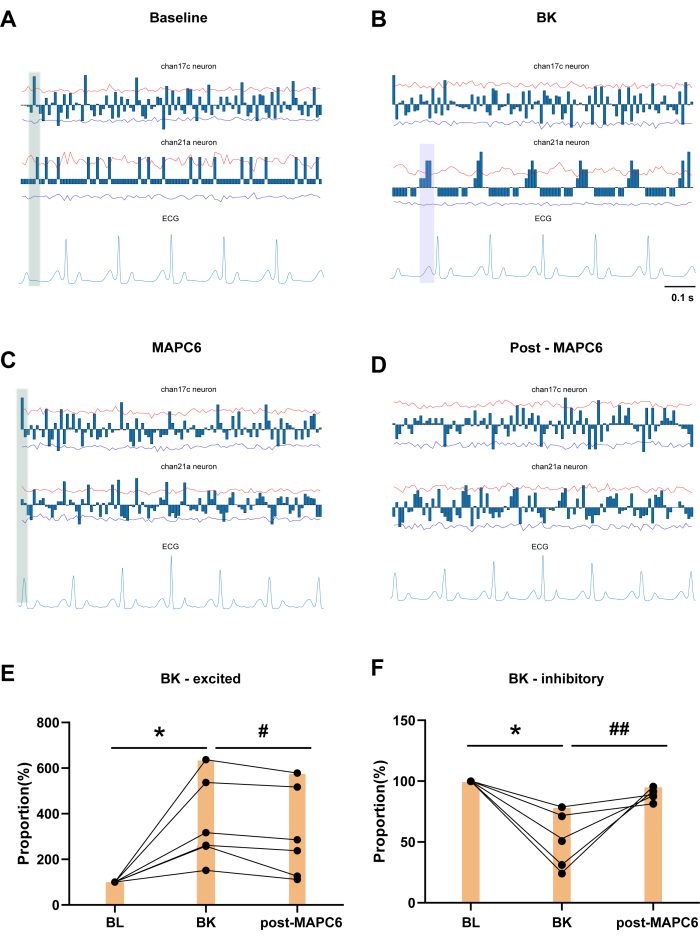
Figure 3: The effect of local BK application to the pericardium on T3 SDHNs and example of cardiac-locked neurons. (A) Channel 17c and channel 21a neurons demonstrate the presence of cardiac-locked neurons in the T3 SDR. (B) Changes in the locking degree after BK administration within 30 min. (C,D) The example of cardiac-locked neuron MAPC6 for 1 min and post MAPC6 for 1 min. (E,F) Change in frequency proportion of neurons responding to BK after MAPC6. Error bars represent mean ± SEM (N = 6). *P < 0.05, compared to BL. #P < 0.05, ##P < 0.01, compared to BK. A paired t-test was used. Abbreviations: BL = baseline; BK = bradykinin; MAPC6= Manual Acupuncture at Neiguan (PC6). Please click here to view a larger version of this figure.
Supplemental File 1: ECG-locked neuronal activity analysis. In line 70: Modify time ranges in condition={[t1_start,t1_end], [t2_start,t2_end], …}. Adjust experimental phase labels in line 71: conditiontitle = {'baseline','EA1','EA2'}; to align with the time ranges defined in condition. Please click here to download this File.
Discussion
Decoding SDH neuronal coding profiles is essential for understanding the neuromodulatory mechanism of acupuncture-induced therapeutic effect on visceral dysfunction. Here, we combined the MEA in vivo recording technique with the ECG recording system to simultaneously record the discharge activity of the T3 SDHNs and the ECG. Cardiac pain stimulation can activate type C nociceptors that innervate the heart and transmit nociceptive information anteriorly through the viscera, DRG, T1-T5 spinal cord, and supraspinal cord. Sympathetic afferent fibers enter the dorsal horn, relay to interneurons, and project to preganglionic neurons in the lateral horn of the spinal cord. These preganglionic fibers enter the white rami communicans and join the sympathetic nerve chain where they are converted into postganglionic neurons directly or upward to the stellate ganglion (SG), and the inferior and middle cervical ganglia synapse with postganglionic neurons to control the heart rate13.
The mechanism of PC6 acupoint in the treatment of cardiovascular diseases is very complex and has not been fully revealed. At present, the somatosympathetic reflex pathway is considered one of the more critical pathways. This study examines the regulatory effects of acupuncture at the PC6 acupoint on cardiac-locked and unlocked neurons within the T3 spinal cord. In the literature, MEA recording techniques have been used to record the discharge of (wide-dynamic range [WDR]) neurons in the SDH14,15. However, the T3 spinal cord is situated close to the ribs and thoracic vertebrae and poses an increased risk of pneumothorax during surgical procedures. As with most in vivo techniques, T3 spinal dorsal horn neurons are not recorded in many experiments. The record of spinal cord stability depends largely on spinal stabilization devices and appropriate anesthesia16. Given that the limitation of commercial spinal clamps, which are inclined to stabilize the lumbar spine, we herein modified the spinal clamp to fit the thoracic spine at first. The modified spinal vertebrae clampers are constructed from a pair of Adson forceps, a metal cap, a round bar, nuts, and screws. After mounting the custom-made clampers to the Narishige STS-B and SR device, we were able to stabilize the rat spine and prevent tissue displacement. Sufficient space was left for the subdorsal horn electrode of the spinal cord.
Although many studies have reported that acupuncture might exert its therapeutic effects on cardiovascular diseases through modulation of hypothalamic, midbrain, and medullar regions, less attention has been paid to the spinal cord17. Furthermore, researchers are used to demonstrating its neuromodulatory mechanism through molecular instead of functional strategies18. Considering the pivotal function of SDHNs in cardiac pathophysiology and their participation in acupuncture's effects, it is imperative to develop a robust analytical method to clarify the scientific mechanisms by which acupuncture influences cardiac function through the modulation of SDHN activity at the functional level. Although Li et al. reported the method of simultaneous recording of T1 dorsal root ganglia neurons calcium imaging and ECG, the time resolution of calcium imaging is low, and the coupling of DRG electrical signal and ECG signal at the millisecond level cannot be detected9.
In terms of SDH electrophysiological recording, Qin et al. recorded SDHN activity and ECG simultaneously19, but the recording flux was low due to the single-electrode recording method. Therefore, in this study, we used MEA electrophysiology combined with ECG recording to obtain high-throughput neuronal discharge signals and ECG signals at the same time. On this basis, we refer to the analysis method for analyzing the degree of coupling between NTS neuron discharge and the ECG signal, and write the analysis code describing the degree of coupling between spinal cord neuron discharge and ECG signal through MATLAB20. Because the remodeling was achieved using commercial forceps, the shape of the clamping arm should be designed following the shape of the thoracic vertebra in the future to reduce the swelling to achieve a better fixing effect. Although this approach provides a powerful tool to study neuronal dynamics, there are also some limitations in measuring large populations of molecularly defined neurons. In the future, the combination of electrophysiological and optical methods may be of great significance to deepen our understanding of the scientific basis of acupuncture.
Interoception and the regulation of the nervous system on internal organs have always been the focus of neuroscience research21,22. Body surface stimulation such as acupuncture can activate physical afferent and modulate nerve activity and visceral function changes23. Studies have shown that cardiac visceral nociceptive information is preliminarily transmitted to the spinal dorsal horn of thoracic segments 1-524. Spinal cord stimulation has also been shown to treat refractory angina pectoris, indicating that modulation of the neural network in the corresponding spinal cord may alleviate cardiac pain. However, to the best of our knowledge, the changes in activity of spinal neurons during cardiac pain and the spinal mechanism underlying peripheral stimulation are not well understood. This study details a method for the concurrent acquisition and analysis of T3 SDHN discharge signals and ECG signals. This method provides significant insights into the regulatory impacts of acupuncture and other peripheral stimuli on cardiovascular neurons in the spinal cord. Moreover, it provides substantial insights into the target organ effects of acupuncture via autonomic neural reflex mechanisms.
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No.82330127, No.82105029), the National Key R&D Program of China (No.2022YFC3500702), and the Fundamental Research Funds for the Central Public Welfare Research Institutes (No. ZZ-2023008) and the Provincial Education Department Project (No. 2019JM-027).
Materials
| Name | Company | Catalog Number | Comments |
| Anesthesia System | Kent Scientific | SomnoSuite | |
| Central v6.5 | Black Microsystems | Cerebus-128 | |
| Fine Scissors | Fine Scissors | Fine Scissors | |
| Friedman-Pearson Rongeurs | Fine Science T ools | 16220-14 | |
| Gelatin Sponges | Coltene | 274-007 | |
| Intubation Cannula | Harward Apparatus | 73-2737 | |
| Isoflurane | RWD | R510 | |
| LabChart Professional Software | LabChart Professional Software | Version 8.0 | |
| microband electrode array | Neuronexus | A1x32-6mm-50-177 | |
| micromanipulator | Narishige | DMA-1510 | |
| needles | Zhongyantaihe | 0.25 mm x 0.25 mm | |
| NeuroExplorer software (V5.0) | Plexon | V5.0 | |
| offline Sorter | Plexon | V4.0 | |
| Powerlab | ADInstruments | PL26T04 | |
| rats | the Experimental Center of the Academy of Military Medical Sciences of the People's Liberation Army of China | ||
| Spinal Adaptor | N/A | N/A | Custom made |
| Spring Scissors | Fine Science Tools | 15023-10 | |
| stereotactic instrument | Narishige | SR-5R-HT |
References
- Nakahara, H., Ueda, S. Y., Kawai, E., Higashiura, R., Miyamoto, T. Effects of pre-exercise acupuncture stimulation on heart rate response during short-duration exercise. BMC Sports Sci Med Rehabil. 13 (1), 129 (2021).
- de Lima Pimentel, R., Duque, A. P., Moreira, B. R., Rodrigues, L. F. J. Acupuncture for the treatment of cardiovascular diseases: A systematic review. J Acupunct Meridian Stud. 12 (2), 43-51 (2019).
- Ma, Q. A functional subdivision within the somatosensory system and its implications for pain research. Neuron. 110 (5), 749-769 (2022).
- Hsieh, M. T., Donaldson, L. F., Lumb, B. M. Differential contributions of A- and C-nociceptors to primary and secondary inflammatory hypersensitivity in the rat. Pain. 156 (6), 1074-1083 (2015).
- Meyr, A. J., Steinberg, J. S. The physiology of the acute pain pathway. Clin Podiatr Med Surg. 25 (3), 305-326 (2008).
- Ardell, J. L. Heart failure: Mechanisms of spinal cord neuromodulation for heart disease. Nat Rev Cardiol. 13 (3), 127-128 (2016).
- Xi, H., et al. Continuous peripheral electrical nerve stimulation improves cardiac function via autonomic nerve regulation in MI rats. Heart Rhythm. 21 (10), 2010-2019 (2024).
- Cui, X., et al. Referred somatic hyperalgesia mediates cardiac regulation by the activation of sympathetic nerves in a rat model of myocardial ischemia. Neurosci Bull. 38 (4), 386-402 (2022).
- Li, X., et al. In vivo thoracic dorsal root ganglia (DRG) calcium imaging and ECG recording for studying reripheral nerve stimulation. J Vis Exp. (210), e67283 (2024).
- Azhar, A., El-Bassossy, H. M. Pentoxifylline alleviates cardiac ischemia and dysfunction following experimental angina in insulin resistance. PLoS One. 9 (5), e98281 (2014).
- Liu, X., Zhang, Q., Han, M., Du, J. Intrapericardial capsaicin and bradykinin induce different cardiac-somatic and cardiovascular reflexes in rats. Auton Neurosci. 198, 28-32 (2016).
- Metropolis, N., Ulam, S. The Monte Carlo method. J Am StatAssoc. 44 (247), 335-341 (1949).
- Coote, J. H., Chauhan, R. A. The sympathetic innervation of the heart: Important new insights. Auton Neurosci. 199, 17-23 (2016).
- Sun, X. Y., et al. Inhibitory effect of acupoint electrostimulation with different layers and intensities on muscular inflammatory pain and spinal WDR neuron activity. Zhen Ci Yan Jiu. 49 (2), 103-109 (2024).
- Duan-Mu, C. L., et al. Electroacupuncture-induced muscular inflammatory pain relief was associated with activation of low-threshold mechanoreceptor neurons and inhibition of wide dynamic range neurons in spinal dorsal horn. Front Neurosci. 15, 687173 (2021).
- Davalos, D., Akassoglou, K. In vivo imaging of the mouse spinal cord using two-photon microscopy. J Vis Exp. (59), e2760 (2012).
- Li, P. Neural mechanisms of the effect of acupuncture. International Congress Series. 1238, 71-77 (2002).
- Wu, X. L., et al. Acupuncture modulation of the ACE/Ang II/AT1R and ACE2/Ang(1-7)/MasR pathways in the rostral ventrolateral medulla reduces sympathetic output and prevents cardiac injury caused by SHR hypertension. Neuroreport. 35 (13), 839-845 (2024).
- Qin, C., Farber, J. P., Foreman, R. D. Gastrocardiac afferent convergence in upper thoracic spinal neurons: a central mechanism of postprandial angina pectoris. J Pain. 8 (6), 522-529 (2007).
- Yao, Y., et al. Cardiovascular baroreflex circuit moonlights in sleep control. Neuron. 110 (23), 3986-3999.e6 (2022).
- Liu, S., et al. Somatotopic organization and intensity dependence in driving distinct NPY-expressing sympathetic pathways by electroacupuncture. Neuron. 108 (3), 436-450.e7 (2020).
- Liu, S., et al. A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature. 598 (7882), 641-645 (2021).
- Ma, Q. Somatotopic organization of autonomic reflexes by acupuncture. Curr Opin Neurobiol. 76, 102602 (2022).
- Wang, J., et al. Spinal cord stimulation reduces cardiac pain through microglial deactivation in rats with chronic myocardial ischemia. Mol Med Rep. 24 (6), 835 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved