Method Article
Isolation and Culture of Primary Cholangiocytes from Mice with Polycystic Liver Disease Using a Two-step Digestion Method
In This Article
Summary
This protocol describes an optimized two-step digestion method for isolating high-purity and high-viability primary cholangiocytes from wild-type mice and mice with polycystic liver disease.
Abstract
In this protocol, we optimized a two-step digestion method to isolate high-purity and high-viability primary cholangiocytes from wild-type mice and mice with polycystic liver disease (PLD). After anesthetizing the mice, the livers were perfused through the inferior vena cava with 50 mL of Solution A, followed by 30 mL of Solution B at 37 °C to enzymatically digest the liver tissue. Mechanical dissociation, shaking, and microdissection were performed to remove adherent parenchymal cells, leaving an intact biliary tree. The biliary tree was then finely minced and digested with shaking for 60 min at 37 °C. The resulting single-cell suspension was collected using a 70 µm cell strainer. Cholangiocytes were purified using immunomagnetic isolation. The cell suspension was incubated with an anti-EpCAM antibody under rotation for 45 min at 4 °C, followed by the addition of Protein G beads and further rotation for another 45 min at 4 °C. After three washes with PBS, the cholangiocytes were collected using a magnetic separator. The purified primary cholangiocytes were resuspended in Cholangiocyte Culture Medium and seeded onto cell culture dishes coated with 1 mg/mL type I rat tail collagen. The purity of the cholangiocytes was confirmed by immunostaining for the cholangiocyte-specific marker cytokeratin-19 (CK19). Although this study focused on isolating primary cholangiocytes from wild-type and PLD mice, we are confident that the protocol can be applied to other disease mouse models as well. This detailed two-step digestion method facilitates in vitro studies of cholangiopathies and the development of targeted therapies.
Introduction
Cholangiocytes, the epithelial cells lining the intrahepatic biliary tree, form a monolayer and constitute approximately 3-5% of the liver's total cell population1. These cells interconnect within the liver to create a complex three-dimensional ductal network2. Under normal conditions, cholangiocytes perform vital functions, including secretion, absorption, injury repair, and serving as an immune barrier, thereby playing a critical role in liver physiology and pathology. However, cholangiocyte dysfunction can lead to various diseases, including PLD, primary sclerosing cholangitis, cholangiocarcinoma, and cholestatic liver injury3,4,5.
Among cholangiopathy-related diseases, PLD stands out as a hereditary disorder marked by the formation of numerous fluid-filled cysts originating from cholangiocytes. The progressive enlargement of these cysts significantly diminishes patients' quality of life6. Current treatment options for PLD remain insufficient, offering limited efficacy while often being associated with high recurrence rates and complications7. This highlights the urgent need to develop safe and effective therapeutic strategies to address the unmet clinical demands in PLD management.
Research into the mechanisms of cholangiopathies, including PLD, has been significantly hindered by the lack of suitable cell lines. To address this limitation, the use of disease mouse models and the isolation of primary cholangiocytes from these models for in vitro experiments have proven invaluable for uncovering the molecular mechanisms underlying cholangiopathies and identifying potential therapeutic strategies.
In our recent study8, we successfully established a PLD mouse model using Pkd1 conditional knockout (KO) mice. Liver cysts were observed as early as 1 month after Pkd1 deletion and progressively increased in size over time. While protocols for isolating cholangiocytes from rats and humans are well-documented9,10, isolating primary cholangiocytes from mice remains particularly challenging due to their small size and the intricate architecture of the portal vein and biliary system. Existing methods face significant limitations, including low cell purity, poor viability, complex procedures, and high costs11,12.
This manuscript presents a detailed protocol for isolating high-purity primary cholangiocytes from mice using a two-step digestion method. This optimized approach aims to support in vitro studies, facilitating the investigation of molecular mechanisms underlying cholangiopathies and advancing the development of novel therapeutic strategies.
Protocol
All mouse care and experimental protocols were approved by the Ethical Committee of Tianjin Medical University (Doc. No: TMUa-MEC 2022016).
1. Preparation of equipment and solutions
- Sterilize surgical instruments (scissors, forceps, and scalpel handles) by autoclaving at least 1 day before cell isolation.
- At least 1 day prior to cell isolation, prepare the Cholangiocyte Culture Medium using the reagents listed in Table 1. Sterilize the Cholangiocyte Culture Medium by filtering it through a 0.22 µm filter.
- At least 1 day prior to cell isolation, prepare Solution A and Solution B using the reagents listed in Table 2 for mouse liver perfusion. Sterilize Solution A and Solution B by autoclaving or filtering through a 0.22 µm filter and adjust their pH to 7.35 using sterile NaOH under gentle stirring.
NOTE: Cholangiocyte Culture Medium, Solution A, and Solution B can be stored at 4°C for at least one month. - Before starting the experiment, add collagenase II powder to Solution B and mix gently to achieve a final concentration of 0.5 mg/mL. Prewarm both Solution A and Solution B in a 37 °C water bath before use.
- Immediately before starting the experiment, prepare 6 cm dishes filled with sterile PBS and place them on ice to keep them cold during the procedure.
2. Liver tissue digestion perfusion
- Anesthetize the mouse using an overdose of isoflurane inhalation. Place the anesthetized mouse in a supine position and secure it with adhesive tape to facilitate the procedure.
- Open the abdominal cavity of the mouse after sterilizing the abdomen with 75% ethanol and then, tie a slipknot around the inferior vena cava using a swaged needle. Rinse a 24 G intravenous catheter with Solution A using a 20 mL syringe.
- Insert the intravenous catheter into the inferior vena cava below the slipknot, secure the slipknot, and fix the catheter in place with adhesive tape.
- Perfuse the liver with 50 mL of Solution A through the inferior vena cava using a 20 mL syringe, and incise the hepatic portal vein to drain the perfusion solution. Maintain the perfusion rate at 10 mL/min.
- Continue perfusion with 30 mL of Solution B containing collagenase II at a flow rate of 3.5 mL/min using a peristaltic pump.
NOTE: This step is intended for the digestion of liver tissue. - Remove the successfully perfused liver tissue and place it in a 6 cm dish filled with cold sterile PBS.
3. Cholangiocytes isolation
NOTE: As the number of cholangiocytes in normal mice is relatively low, it is recommended to use a group of 2-6 mice for the procedure. However, for mouse disease models with abnormal cholangiocyte proliferation, such as PLD, the cholangiocytes from a single mouse are sufficient. Unless otherwise specified, samples and reagents need always be kept on ice, and all procedures should be performed in a biological safety cabinet.
- Mechanically dissociate and shake the liver tissue using curved forceps. Subsequently, perform microdissection under a dissecting microscope to remove adherent parenchymal cells, leaving an intact biliary tree. Remove the gallbladder tissue prior to proceeding to the next step.
Wash the tissue 2x with PBS. - Finely mince the biliary tree using a surgical blade, and digest it in 3 mL of Digestion Solution (Table 3) per mouse with shaking (90 rpm) at 37 °C for 60 min. At the 30 min mark, gently pipette the mixture using a pipette tip to mix thoroughly.
NOTE: The Digestion Solution should be freshly prepared before use. - Pass the cell suspension through a 70 µm cell strainer. Use the plunger of a 5 mL or 10 mL syringe to gently press the cell suspension through the strainer. Wash the strainer with PBS.
- Centrifuge the cell suspension at 500 x g for 5 min at 4 °C. Wash the cells 3x with RPMI medium.
- Centrifuge again at 500 x g for 5 min at 4 °C.
- Discard the supernatant and resuspend the cells in 900 µL of RPMI medium containing 40 U/mL DNase I. Add 2 µg of anti-EpCAM antibody and incubate the suspension on a rotator (20 rpm) at 4 °C for 45 min.
- Centrifuge at 500 x g for 5 min at 4 °C.
- Discard the supernatant and resuspend the cells in 1 mL of RPMI medium containing 40 U/mL DNase I. Prewash Protein G beads with 1 mL of RPMI medium. Add 20 µL of Protein G beads to the cell suspension and incubate with rotation (20 rpm) at 4 °C for another 45 min.
NOTE: The anti-EpCAM antibody can also be preincubated with Protein G beads to create a bead-antibody complex prior to introducing the cell suspension. - Wash the cells 3x with PBS using a magnetic separator.
- Perform cell counting and cell viability assessment using Trypan Blue staining (0.04%, 3 min) on a hemocytometer.
NOTE: There is no need to remove the beads from the cholangiocytes, they will detach naturally once culturing begins and will not interfere with cell culture.
4. Cholangiocytes culture
NOTE: Prepare fresh type I rat tail collagen-coated cell culture dishes prior to cholangiocytes culture. Prechill all reagents on ice.
- Following the manufacturer's instructions, mix sterile ddH2O, 10x PBS, 1 N NaOH, and type I rat tail collagen to achieve a final concentration of 1 mg/mL type I rat tail collagen in 1x PBS. Evenly coat the cell culture dishes with the mixture, and place the dishes in an incubator at 37 °C with 5% CO2 for 30 min to allow the collagen to solidify.
- Wash the type I rat tail collagen-coated cell culture dishes with prewarmed PBS.
- Resuspend the cholangiocytes in prewarmed Cholangiocyte Culture Medium. Seed the cell suspension onto 1 mg/mL type I rat tail collagen-coated cell culture dishes and incubate at 37 °C with 5% CO2. Replace the medium every 2 days.
5. Passaging and cryopreservation of cholangiocytes
- Wash cholangiocytes twice with PBS, then incubate the cells in 0.25% trypsin at 37 °C for 5-10 min.
- Add an equal volume of Cholangiocyte Culture Medium to neutralize the trypsin, then centrifuge at 500 x g for 5 min.
- Prepare type I rat tail collagen-coated cell culture dishes as described in Step 4.1.
- Follow steps 4.2 and 4.3.
- If cryopreservation is required, resuspend the cholangiocytes in Storage Medium (9:1 FBS: DMSO) and store in a -80 °C freezer or liquid nitrogen. Thaw the cells as needed for future use.
6. Cholangiocyte validation
NOTE: The purity of cholangiocytes was assessed by staining for cholangiocyte marker CK19.
- Wash the cholangiocytes 2x with PBS, then fix the cells in 4% paraformaldehyde for 15 min.
- Wash the cholangiocytes with PBS, then permeabilize the cells with 1% Triton X-100 for 8 min.
- Wash the cholangiocytes with PBS, then block the cells in 5% BSA for 1 h.
- Wash the cholangiocytes with PBS, then incubate the cells with the primary antibody against CK19 (dilution 1:12 with 2% BSA) overnight at 4 °C.
- Wash the cholangiocytes 2x with PBS, then incubate the cells with donkey anti-rat Alexa Fluor 488 secondary antibody (dilution 1:1,000 with PBS) for 1 h at room temperature.
- Wash the cholangiocytes with PBS, stain with 4',6-diamidino-2-phenylindole for 15 min, and observe under a microscope.
Results
The workflow diagram for the two-step digestion process used to isolate cholangiocytes is shown in Figure 1. The entire procedure takes ~5 h. First, the liver is perfused with Solution A through the inferior vena cava to remove blood, as indicated by the liver turning pale. The liver is then perfused with Solution B containing collagenase II to initiate tissue digestion. This initial digestion step is time-sensitive, and successful digestion is evidenced by the tissue becoming noticeably soft. Next, mechanical dissociation and gentle shaking using curved forceps help remove hepatocytes; proper digestion is confirmed when a large number of hepatocytes detach. Under a dissecting microscope, the remaining adherent parenchymal cells are carefully removed by microdissection, leaving an intact biliary tree (Figure 2).
In the second digestion step, the tissue is broken down into a single-cell suspension, which still contains some hepatocytes and fibroblasts. Finally, high-purity cholangiocytes are obtained from this suspension through immunomagnetic separation. Cell viability assessment was conducted using Trypan Blue staining on a hemocytometer. The results demonstrated the successful isolation of highly viable cholangiocytes (Figure 3). Immunofluorescence staining for CK19 (Figure 4) confirmed that this method consistently yields high-purity mouse cholangiocytes.
Primary cholangiocytes typically adhered to the culture surface and formed a monolayer within approximately 24 h, exhibiting rapid proliferation during the first three passages, especially in mouse disease models with abnormal cholangiocytes proliferation, such as PLD (Figure 5). However, at later passages, the growth rate decreased, and the cells became larger and developed a vacuolated morphology. There is no need to remove the beads from the cholangiocytes, they will detach naturally once culturing begins and will not interfere with cell culture. Cell cryopreservation and thawing did not significantly affect cell viability.

Figure 1: Schematic overview of the two-step digestion process for cholangiocyte isolation. This figure was taken from Ji et al8. Please click here to view a larger version of this figure.

Figure 2: Representative image of the biliary tree isolated from a mouse with polycystic liver disease. Please click here to view a larger version of this figure.

Figure 3: Cell viability assessment of isolated cholangiocytes using Trypan Blue staining. Scale bars = 50 µm. The small black particles surrounding the cells and within the background are Protein G beads. Please click here to view a larger version of this figure.

Figure 4: Cell purity assessment of isolated cholangiocytes using immunofluorescence staining for CK19. Scale bars: = 50 µm [upper] and 10 µm [lower]. This figure was taken from Ji et al8. Please click here to view a larger version of this figure.
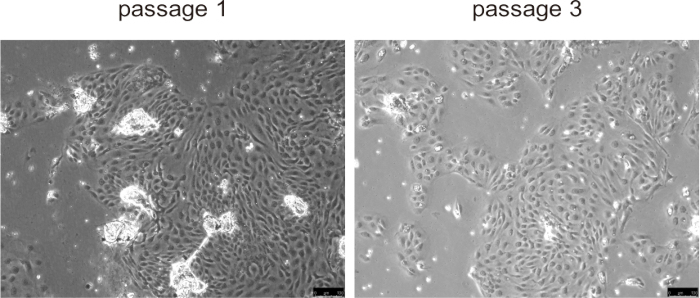
Figure 5: Representative images of isolated cholangiocytes in passage 1 and passage 3. Scale bars = 100 µm. Please click here to view a larger version of this figure.
| Components | Amount |
| DMEM/F12 (1:1) | 415 mL |
| Fetal Bovine Serum (FBS) | 50 mL |
| MEM non-essential amino acids (100x) | 5 mL |
| Insulin-transferrin-selenium (100x) | 5 mL |
| MEM vitamin solution (100x) | 5 mL |
| Chemically-defined lipid concentrate (100x) | 5 mL |
| Penicillin-Streptomycin (100x) | 5 mL |
| Gentamicin/amphotericin solution (500x) | 1 mL |
| 100 mM Na Pyruvate | 250 μL |
| 10 mg/mL Soybean trypsin inhibitor | 2.5 mL |
| 200 mM L-Glutamine | 5 mL |
| 10 mg/mL Dexamethasone | 20 μL |
| 1.7 mg/mL 3,3',5-triiodo-L-thyronine | 1 mL |
| 1 mg/mL Epidermal growth factor | 12.5 μL |
| 20 mM Forskolin | 250 μL |
| Ethanolamine | 13 μL |
Table 1: Composition of Cholangiocyte Culture Medium.
| Components | Amount | |
| Solution A | NaCl | 4.0908 g |
| KCl | 0.1578 g | |
| Na2HPO4 | 0.1072 g | |
| HEPES | 2.9789 g | |
| 50 mM EGTA (PH = 8) | 5 mL | |
| 1.5 M MgCl2 | 34 μL | |
| ddH2O | 500 mL | |
| NOTE: Adjust the pH to 7.35 using sterile NaOH. | ||
| Solution B | NaCl | 4.0908 g |
| KCl | 0.1578 g | |
| Na2HPO4 | 0.1072 g | |
| HEPES | 2.9789 g | |
| 2 M CaCl2 | 1.25 mL | |
| ddH2O | 500 mL | |
| NOTE: Adjust the pH to 7.35 using sterile NaOH. Collagenase II powder should be added to achieve a final concentration of 0.5 mg/mL before use. | ||
Table 2: Composition of Solution A and Solution B.
| Components | Amount |
| 3.2 mg/mL collagenase XI | 300 μL |
| 10 mg/mL hyaluronidase | 120 μL |
| 10 U/μL DNase I | 12 μL |
| Penicillin-Streptomycin (100x) | 30 μL |
| RPMI medium | 2.538 mL |
Table 3: Composition of Digestion Solution.
Discussion
This protocol provides a detailed method for isolating high-purity primary cholangiocytes from mice using a two-step digestion process, enabling the study of the molecular mechanisms underlying cholangiopathies. Several critical steps are essential to ensure the successful isolation of cholangiocytes.
The first critical step is ensuring effective perfusion and digestion using Solution A and Solution B. Successful perfusion with Solution A was confirmed by the liver turning pale, and successful digestion with Solution B was confirmed by the liver tissue becoming noticeably soft. Failure in perfusion would impede liver tissue digestion and compromise subsequent isolation of the biliary tree. To enhance digestion efficiency, one could increase the volumes of Solution A and Solution B, reduce the perfusion flow rate, or gently press the liver with a cotton swab.
The second critical step involves mechanically dissociating and gently shaking the liver tissue with curved forceps, followed by microdissection under a dissecting microscope to remove adherent parenchymal cells and isolate an intact biliary tree. This step was key to obtaining high-purity cholangiocytes. Efficiency could be improved by grasping larger bile ducts with curved forceps during shaking and rinsing with cold PBS. If the liver is digested successfully, many hepatocytes will detach, making microdissection relatively straightforward. However, if digestion is incomplete and the sample is valuable, the microdissection process will require painstaking effort, increasing workload and potentially reducing both cell yield and purity. Thus, ensuring proper perfusion with Solution B for liver digestion was paramount.
The third critical step is the second digestion of the biliary tree to produce a single-cell suspension. Digestion time should be optimized by observing the cells under a microscope: overdigestion may reduce cell viability, while underdigestion could lead to unused biliary tissue. Additionally, including appropriate antibiotics in all reagents helps minimize contamination.
Using this optimized two-step digestion protocol, we successfully isolated high-purity and highly viable cholangiocytes from mice. While this study focused on isolating primary cholangiocytes from wild-type and PLD mouse models, we are confident that the protocol can be applied to other disease mouse models as well. The process of initial liver tissue digestion to isolate the biliary tree, followed by a second digestion step, significantly improved the efficiency of immunomagnetic separation and yielded a higher number of viable cells. The isolated cholangiocytes were well-suited for epigenetic experiments requiring high viability, such as ATAC-seq, ChIP-seq, low-input ChIP-seq, and CUT&TAG. Additionally, these primary cholangiocytes could be cultured in vitro for various functional assays, including 3D cystic growth, cell proliferation, apoptosis, and cell cycle analysis.
In a previously published study8, we isolated both normal and cystic primary cholangiocytes from wild-type and PLD mice of both sexes. Multi-omics profiling of primary cholangiocytes from male and female PLD mice revealed sex-specific epigenetic dynamics in cholangiocytes during hepatic cystogenesis. Furthermore, we identified a potential epigenetic therapeutic strategy for male PLD patients through pharmacological inhibition of epigenetic modifying enzymes in both PLD mouse models and in vitro experiments with primary cholangiocytes.
Our approach offers several notable advantages over currently published cholangiocyte isolation methods. Compared to the method of Kudira et al.13, which involves directly digesting liver tissue into a single-cell suspension after perfusion digestion, followed by gradient centrifugation and immunomagnetic isolation, our method is simpler, more cost-effective, and more efficient. A key advantage is the isolation of the biliary tree prior to immunomagnetic isolation, which improves the overall process. Additionally, by not requiring the removal of the beads from the cholangiocytes, this method achieves a higher yield and enhanced viability of the isolated cells. In comparison to the method of Ueno et al.14, which dissociates and minces the portal tract residue from liver digestion perfusion for 3D culture, followed by a 7 day culture period before generating a single-cell suspension, our method is more time-efficient. The biliary tree isolation, followed by immunomagnetic isolation, also significantly enhances cell purity.
However, despite these advantages, our two-step digestion technique still has some limitations. It is relatively labor-intensive, allows for only a limited number of passages, and requires careful microdissection to avoid parenchymal contamination.
In conclusion, we developed and optimized a two-step digestion method for isolating high-purity and high-viability primary cholangiocytes from mice. This approach provides a valuable tool for studying the molecular mechanisms underlying cholangiopathies and offers a foundation for identifying potential therapeutic strategies.
Disclosures
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by grants from the Tianjin Municipal Education Commission (2022ZD054 to L.Z.)
Materials
| Name | Company | Catalog Number | Comments |
| 0.22 μm filter | PALL | 4612 | |
| 0.25% Trypsin | Gibco | 25200-056 | |
| 10 mL syringe | KONSMED | 10 mL 1.2*30TWLB | |
| 20 mL syringe | KONSMED | 20 mL 1.2*30TWLB | |
| 24 G intravenous catheter | WEGO | 24GX19 mm/Y-G | |
| 3,3',5-triiodo-L-thyronine | Sigma | T5516 | 1.7 mg/mL stock |
| 4% paraformaldehyde | Solarbio | P1110 | |
| 5 mL syringe | KONSMED | 5 mL 0.7*30TWLB | |
| 6 cm dishes | Thermo Scientific | 150462 | |
| 70 µm cell strainer | Corning | 352350 | |
| anti-CK19 antibody | DSHB | TROMA-III | |
| anti-EpCAM antibody | DSHB | G8.8 | |
| BSA | Solarbio | A8020 | |
| CaCl2 | Sangon Biotech | A501330 | 2 M stock |
| Chemically-defined lipid concentrate (100x) | Gibco | 11905-031 | |
| Collagenase II | Worthington | LS004176 | |
| Collagenase XI | Sigma | C7657 | 3.2 mg/mL stock |
| Dexamethasone | Sigma | D1756 | 10 mg/mL stock |
| Dissecting Microscope | Leica | EZ4 | |
| DMEM/F12 (1:1) | VivaCell biosciences | C3130-0500 | |
| DMSO | Sigma | D2650 | |
| DNase I | Sigma | D4513 | 10 U/μL stock |
| Donkey anti-rat Alexa Fluor 488 secondary antibody | Invitrogen | A21208 | |
| EGTA | Solarbio | E8050 | 50 mM (PH = 8) stock |
| Epidermal growth factor (1 mg/mL) | Sigma | SRP3196 | |
| Ethanolamine | Sigma | E9508 | |
| Fetal Bovine Serum | VivaCell biosciences | C04001-500 | |
| Forskolin | Sigma | F3917 | 20 mM stock |
| Gentamicin/amphotericin solution (500x) | Gibco | R01510 | |
| Hemocytometer | QIUJING | XB.K.25. | |
| HEPES | Sigma | H4034 | |
| Hyaluronidase | Sigma | H3506 | 10 mg/mL stock |
| Insulin-transferrin-selenium (100x) | Gibco | 41400045 | |
| Isoflurane | RWD | R510-22-10 | |
| KCl | Sangon Biotech | A100395 | |
| L-Glutamine (200 mM) | Sigma | G7513 | |
| Magnetic separator | Promega | Z5342 | |
| MEM non-essential amino acids (100x) | Gibco | 11140050 | |
| MEM vitamin solution (100x) | Gibco | 11120052 | |
| MgCl2 | Sangon Biotech | A100288 | 1.5 M stock |
| Na Pyruvate (100 mM) | Gibco | 11360070 | |
| Na2HPO4 | Sangon Biotech | A600487 | |
| NaCl | Sangon Biotech | A610476 | |
| NaOH | Sangon Biotech | A620617 | 1 N stock |
| PBS | VivaCell biosciences | C3580-0500 | |
| PBS | Solarbio | P1010 | |
| Penicillin-Streptomycin (100x) | Gibco | 15140-122 | |
| Peristaltic pump | BaodingRongbai | YZ1515PPS | |
| Protein G beads | Invitrogen | 10004D | |
| Rotator | Kylin-Bell | QB-528 | |
| RPMI | VivaCell biosciences | C3010-0500 | |
| Soybean trypsin inhibitor | Sigma | T6522 | 10 mg/mL stock |
| Swaged needle | Jinhuan Medical | HM601 | |
| Triton X-100 | Solarbio | T8200 | |
| Trypan blue | Sigma | T6146 | 10 mg/mL stock |
| Type I rat tail collagen | BD | 354236 |
References
- Wang, Z., et al. Human cholangiocytes form a polarized and functional bile duct on hollow fiber membranes. Front Bioeng Biotechnol. 10, 868857 (2022).
- Li, P., et al. Three-dimensional human bile duct formation from chemically induced human liver progenitor cells. Front Bioeng Biotechnol. 11, 1249769 (2023).
- Fabris, L., et al. Pathobiology of inherited biliary diseases: a roadmap to understand acquired liver diseases. Nat Rev Gastroenterol Hepatol. 16 (8), 497-511 (2019).
- Jalan-Sakrikar, N., et al. Central role for cholangiocyte pathobiology in cholestatic liver diseases. Hepatology. , (2024).
- Ilyas, S. I., Gores, G. J. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 145 (6), 1215-1229 (2013).
- Perugorria, M. J., et al. Polycystic liver diseases: advanced insights into the molecular mechanisms. Nat Rev Gastroenterol Hepatol. 11 (12), 750-761 (2014).
- Masyuk, T. V., Masyuk, A. I., LaRusso, N. F. Polycystic liver disease: Advances in understanding and treatment. Annu Rev Pathol. 17, 251-269 (2022).
- Ji, R., et al. Multi-omics profiling of cholangiocytes reveals sex-specific chromatin state dynamics during hepatic cystogenesis in polycystic liver disease. J Hepatol. 78 (4), 754-769 (2023).
- Tabibian, J. H., et al. Characterization of cultured cholangiocytes isolated from livers of patients with primary sclerosing cholangitis. Lab Invest. 94 (10), 1126-1133 (2014).
- Muff, M. A., et al. Development and characterization of a cholangiocyte cell line from the PCK rat, an animal model of autosomal recessive polycystic kidney disease. Lab Invest. 86 (9), 940-950 (2006).
- Nagaya, M., Katsuta, H., Kaneto, H., Bonner-Weir, S., Weir, G. C. Adult mouse intrahepatic biliary epithelial cells induced in vitro to become insulin-producing cells. J Endocrinol. 201 (1), 37-47 (2009).
- Karjoo, S., Wells, R. G. Isolation of neonatal extrahepatic cholangiocytes. J Vis Exp. (88), e51621 (2014).
- Kudira, R., et al. Isolation and culturing primary chaolangiocytes from mouse liver. Bio Protoc. 11 (20), e4192 (2021).
- Ueno, Y., et al. Evaluation of differential gene expression by microarray analysis in small and large cholangiocytes isolated from normal mice. Liver Int. 23 (6), 449-459 (2003).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved