Method Article
In Vitro Evaluation of The Effects Of Er,Cr:YSGG and Diode Lasers Used on Titanium Cylinder
In This Article
Summary
In this study, Er,Cr:YSGG and diode lasers applied separately to the flat surface of a total of 96 specially designed titanium cylinders. A thermocouple placed on the other surface and the temperature was measured. Surface roughness analyzed by profilometer, SEM and AFM.
Abstract
Peri-implant diseases are significant issues associated with dental implants. The aim of this study was to evaluate the surface roughness and temperature changes when diode and Erbium, Chromium-doped Yttrium-Scandium-Gallium-Garnet (Er,Cr:YSGG) lasers are applied on titanium cylinders in the treatment of non-surgical peri-implant diseases.A total of 13 groups, including the control group, were formed with 940 nm diode (0.8 W-1.3 W-1.8 W) and Er,Cr:YSGG (1.5 W-2.5 W-3.5 W) lasers in 6 different power modes, 20s/W and 40 s/W, and eight titanium cylinders were treated in each group. During the process, the initial and final temperatures were recorded with a thermocouple placed in the apical slot of the cylinder. After the application, the roughness (Ra) of all disks was measured by a profilometer. The surfaces were scanned by scanning electron microscope (SEM) and atomic force microscope (AFM) for two- and three-dimensional surface examination.When the temperature changes of the titanium cylinders during irradiation were evaluated, the cylinders irradiated with a diode laser for 40 s were significantly higher than those irradiated for 20 s. In the Er,Cr:YSGG treated cylinders, the temperature decreased in some samples and increased minimally in some samples. Profilometer values (Ra) were not statistically significant in terms of roughness for all groups. However, SEM images showed melting and an increase in the number of micropores on the treated surfaces.With the limitations of this in vitro study, the application of the Er,Cr:YSGG, and the diode can be regarded as a safe approach for the management of peri-implantitis, particularly in terms of thermal safety. While the surface roughness remains unchanged, the use of these lasers resulted in melting changes and micropores on the Ti cylinder topography. To determine how these laser settings affect bacterial decrease and osseointegration, additional research is needed.
Introduction
Dental implants are a commonly accepted treatment option for the replacement of lost teeth1,2. Peri-implant mucositis and peri-implantitis are classified as peri-implant diseases. Peri-implant mucositis is restricted to soft tissues, and there is no evidence of bone loss, with the exception of physiological bone remodeling. Peri-implantitis is a pathological condition that is associated with plaque and affects the tissues surrounding dental implants. It is distinguished by the inflammation of the peri-implant mucosa and the consequent increasing loss of supporting bone3. The primary etiological factor for the initiation and progression of the disorder is disruption of the peri-implant plaque biofilm4. Numerous studies on peri-implant illnesses indicate that the prevalence of peri-implant mucositis (PIM) ranges from 9.7% to 64.6%, while the prevalence of peri-implantitis (P) varies between 4.7% and 45%5.
While plaque accumulation is the main etiological factor that causes peri-implantitis, its treatment is complicated by the diverse topographical characteristics of implants. The foundation of nonsurgical peri-implantitis treatment is infection management through the debridement of the implant surface and the elimination of adhering biofilm to decrease bacterial load below the disease-causing threshold6,7. The complex micro and macro-topography of titanium interfaces and bone defect anatomy limits surface decontamination. The efficacy of different mechanical (curettes, ultrasonic devices, air-powder abrasion, titanium brushes), chemical (citric acid, chlorhexidine, antimicrobials), and physical (laser, photodynamic therapy) decontamination techniques have been assessed in combination8. Current research suggests that the combined use of non-surgical intervention techniques for peri-implantitis is more effective than debridement alone9. The incorporation of chemical antimicrobial agents or local/systemic antibiotics into mechanical therapy has demonstrated significant efficacy; nevertheless, these interventions could result in possible adverse consequences10. As laser technology has advanced, dental lasers have become increasingly popular because of their anti-infective, detoxifying, and user-friendly effects on implant surfaces10,11.
The absorption peak, operational mode of the device, and tissue properties affect the heat increase during laser irradiation. A crucial pre-clinical investigation revealed that an elevation in temperature to 50 °C for 1 min caused vascular damage, whereas a rise to 60 °C led to the cessation of blood flow and subsequent bone necrosis12. An in vitro investigation found that after just 10 s of diode laser irradiation, implant surfaces could reach temperatures higher than the bone safety threshold (10 °C). Bone viability could be compromised by a temperature increase of just 10 °C13.
Numerous recent studies have concentrated on examining the beneficial impact of lasers in this domain14,15,16,17,18. Various laser wavelengths demonstrate a significant antibacterial impact and safety on implant surfaces when appropriate parameters are applied. A number of variables, including intensity, frequency, and wavelength, influence the efficacy of laser treatments. Several studies have demonstrated the bactericidal effect of various laser wavelengths, including CO2, Er:YAG, Er,Cr:YSGG and various diode lasers, which allows us to identify the beneficial effects of different lasers in the treatment of peri-implantitis. Aoki et al19,20,21. concluded from their review that laser application facilitates surface cleaning in both non-surgical and surgical peri-implant treatments, including regenerative therapy, and promotes healing by activating surrounding tissue cells22.
Diode lasers have the ability to exert a bactericidal effect on implant surfaces without affecting the implant's surface pattern. When it comes to treating peri-implantitis, the diode laser may be the way to go because it promotes the healing of periodontal tissues23,24,25.
Erbium, chromium-doped: yttrium, scandium, gallium, garnet (Er,Cr:YSGG) lasers exhibit effective properties for the elimination of biofilm and the decontamination of implant surfaces11. Strong bactericidal effects and bone regeneration properties were demonstrated by erbium lasers without causing mechanical damage thanks to their water-powered properties11,14.
There is a shortage of data regarding the alterations caused by laser irradiation on titanium implants. Moreover, a definitive methodology for the irradiation of titanium surfaces has yet to be defined, encompassing laser parameters such as power and time of application. Previous studies showed that Er,Cr:YSGG laser16 application had no effect on temperature change, however, diode laser studies exceeded13 and did not exceed16,26 the critical value. Different results of the effect of laser treatment on the Ra value of the titanium surface are available in the literature18,27. The null hypothesis of the study is that there will be no difference between Er,Cr:YSGG lasers, and diode lasers in terms of temperature and roughness change of titanium surfaces by using. This study aimed to determine safe operating parameters by monitoring surface roughness and temperature variations on titanium material using Er,Cr:YSGG, and diode lasers at various time and power settings. The evaluation of temperature change was conducted with a thermocouple, surface roughness was assessed using a profilometer, and surface alterations were analyzed through SEM and AFM techniques.
Protocol
NOTE: Titanium cylinders, crafted from the same material as conventional implants and designed to replicate the implant surface with SLA technology, feature a height of 10 mm and a diameter of 5 mm. A cavity measuring 7mm in depth and 3mm in width is located at the center of the cylinders (Fig. 2). The width of 3mm reduces to 1mm at the deepest point. Measuring the surface roughness of standard implants with a profilometer is not feasible. It was possible to assess the effectiveness of the laser applied to a 5 mm diameter flat surface at the top of the titanium cylinder designed by the manufacturer, utilizing the same material that simulates the implant surface. Additionally, in order to measure temperature changes from the center of the cylinder, a groove 7 mm deep and 3 mm wide has been created from the center of the bottom surface of the titanium cylinder towards the depths of the cylinder, where the thermocouple tip will be placed. This groove allows for the evaluation of the temperature change of the treated surface from within the cylinder, rather than dependent on the outer surface. Three-dimensional visuals were obtained by analyzing the flat surfaces of specially produced titanium cylinders using an Atomic Force Microscope (AFM). A 940 nm diode (0.8 W28, 1.3 W29, 1.8 W30) and 2.780 nm Er,Cr:YSGG (1.5 W31, 2.5 W31, 3.5 W32) lasers were used at three different wattages according to company recommendations, and 12 groups were formed with 20 s and 40 s application time each. After the application, a control group was added for roughness evaluation. A stand with a finger support was printed from a three-dimensional printer to keep the Ti cylinder stable during the application (Table of Materials).
1.Sample size
- Calculate sample size using the Power analysis of the G*Power program. The minimum number of samples for each group was determined as n=8 samples for temperature change with effect size d: 0.6906, standard deviation 16.8, Power: 0.80, and α: 0.05.
NOTE: In this study, an Er,Cr:YSGG laser equipped with a 940 nm diode laser with a 300 µm diameter tip (e3 tip) and a 360° firing elastic RFPT5-14 tip (580 µm diameter and 14 mm long) was used as the laser system (Figure 1).

Figure 1: Instruments and equipment used. (A) Diode laser, (B) Er,Cr:YSGG laser, (C) E3 tip, (D) RPTF5-14 tip. Please click here to view a larger version of this figure.
2. Determination of working groups
- Take the recommended Watt of lasers for in-pocket application in peri-implant mucositis as the ideal condition of use. Additionally, include one lower value and one higher value in the study groups relative to the recommended Watt. The values used here are 1.5 W and 3.5 W for Er,Cr:YSGG, and 0.8 W and 1.8 W for the diode laser.
- Determine application time as 20 s and 40 s to evaluate the effect of operating time on temperature change. Study groups are shown in Table 1.
| Group Name | Laser Type | Number of Samples (n) | Watt (W) | Time (s) |
| E1 | Er,Cr:YSGG | 8 | 1.5 W | 20 |
| E2 | 8 | 2.5 W | 20 | |
| E3 | 8 | 3.5 W | 20 | |
| E4 | 8 | 1.5 W | 40 | |
| E5 | 8 | 2.5 W | 40 | |
| E6 | 8 | 3.5 W | 40 | |
| D1 | Diode | 8 | 0.8 W | 20 |
| D2 | 8 | 1.3 W | 20 | |
| D3 | 8 | 1.8 W | 20 | |
| D4 | 8 | 0.8 W | 40 | |
| D5 | 8 | 1.3 W | 40 | |
| D6 | 8 | 1.8 W | 40 | |
| C | Control | 8 |
Table 1: Study groups information.
3. Preparation of experimental setup
- With the Rhinoceros (3D graphics and design) program, design a cylinder stand in 3D with a cavity slightly with a diameter of 10 mm and a thickness of 5 mm.
- Open the app. Draw a circle 10 mm in diameter. Reduce the circle by 50% from one axis to create an ellipse. Press Extuder and raise the ellipse in the third dimension.
- Draw a circle again for finger support. Raise the second circle in the third dimension with the extruder key. Make the height less than the first circle.
- Drill a 10 mm hole in the elliptical drawing with the Boolean command. For thermocouple support, make an L-shaped line with the Sweep 1 command and create the 3rd dimension.
- Draw a square, enlarge it in the 3rd dimension with the extruder command, and create the base. After printing the design, apply silicone around the hole where the Ti cylinder will come and dry it. This will keep the cylinder in place while the laser is being applied.
- Bring a closed room with air conditioning to a temperature of 27 °C. Fix the stand on which the cylinders will be fixed in the middle of a plastic tub with double-sided tape.
NOTE: Insert Ti Cylinder, squeeze air onto the surface. - Place the thermocouple of the thermometer in the hollow part of the Ti cylinder placed in its slot on the stand.
- Prepare a chronometer to keep track of the application time. Record the degrees by the 3rd observer and keep track of the time with stopwatch.
4. Experimental procedure
- During laser application, wear protective glasses for practitioner safety.Insert RPTF5-14 tip for Er,Cr:YSGG laser. Insert tip E3 for diode laser.
- Turn on the Er,Cr:YSGG laser.Select Perio Closed Mode. Apply 1.5 W, 2.5 W and 3.5 W lasers for 20 s and 40 s each. There are 96 Ti cylinders with laser applied. Irradiate a Ti cylinder with only one laser type, one watt and one time.
- Turn on the diode laser. Select Perio Pocket Mode. Apply 0.8 W, 1.3 W and 1.8 W laser for 20 s and 40 s each.
- Have the 3rd observer start the timer when the laser starts. Warn him/her when the time is up.
- Apply the laser tip at a 15° angle to the surface, in contact, zigzagging over the entire surface for the planned time.
- Note the initial and final temperature values during the application. Subtract the start temperature value from the end temperature value. Calculate the temperature change.
NOTE: Note the temperature change values for a total of 12 groups, 6 Er,Cr:YSGG, and 6 Diode laser groups. - Keep samples in transparent bags with group numbers written on them.
5.Two and Three-dimensional imaging of materials
- Perform scanning electron microscope (SEM) and atomic force microscope (AFM) analyses to evaluate and demonstrate changes in the morphology of the Ti cylinder surface.
NOTE: FEI Quanta FEG 250 instrument was used. - Do not coat samples before being placed in the SEM. There are 13 groups, including 1 control group, 6 diode laser groups, and 6 Er,Cr: YSGG laser groups. For the control group, perform no treatment, only take surface images by AFM and SEM.
- Randomly select one cylinder from each of the 13 study groups. Insert them into the SEM device.Note the location on the platform and the sample code to avoid mixing samples.
- Place the Ti cylinder in the SEM device with the flat surface upwards. Perform analyses using Low vacuum mode. Set the chamber pressure to 60 Pa during analysis.
- Once the device is fully ready, record images at 250x, 1000x, and 5000x magnification from a random point on the flat surface.Repeat this procedure for all samples.
NOTE: When the SEM device finishes vacuuming, it is ready for image collection. - For AFM measurement, randomly select one Ti cylinder from each study group. Perform measurement in tapping mode.
- Place the Ti cylinder in the AFM instrument. Place the top cover so that the tip of the instrument is over the sample. Check that the red light from the window on the instrument is on the surface to be imaged.
- Set the voltage to 2. Move the tip closer to the sample with the Auto land button. Start the scan by pressing the Start Scan button.
- Take a 5 µm x 5 µm digital image for each sample and record at a slow scan rate (1 Hz). Record images taken with the AFM instrument from the flat surfaces of Ti cylinders. AFM instrument visualizes 25 µm2 area.
6. Measurement of surface roughness
NOTE: The Surftest SJ 201, Mitutoyo, Tokyo, Japan, device was used here.
- Set the resolution of the profilometer to 0.01 mm, the transverse length to 3.0 mm and the diameter of the diamond recording pin tip to 5 µm. Set the measurement speed to 0.5 mm/s to determine the Ra value.
- Fix the Ti cylinder with a holder, use the presel, and fix the Ti cylinder by holding it from the side surface. Place the needle of the profilometer in contact with the Ti surface.
- Press the Start button. Save the Ra value found. Repeat the measurement 5x in different directions on the flat surface of each cylinder (Figure 2). To achieve the various directions, move the Ti cylinder around itself with the help of a presell. Repeat for the entire length of the Ti cylinder.
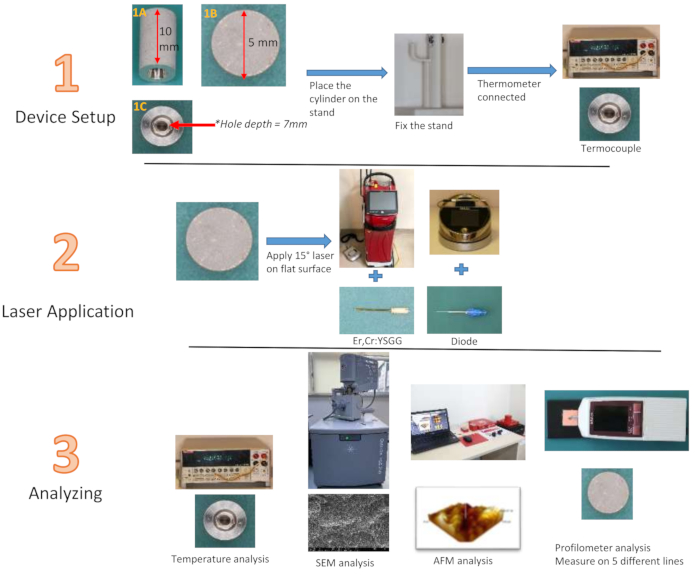
Figure 2: Flowchart of study groups. (1A) Ti cylinder side view, (1B) top view, (1C) bottom view Please click here to view a larger version of this figure.
7. Statistical analysis
- Perform statistical analysis using the SPSS-Windows statistical package program and apply the Kruskal-Wallis and Mann-Whitney tests. Set statistical confidence level at 95% (α = 0.05).
Results
Upon evaluation based on the application times of 20 seconds and 40 seconds, a statistically significant difference was observed. The temperature change on the 40 s laser applied Ti cylinder surfaces was observed to be greater than that on the 20 s laser applied (p=0.037; Figure 3).
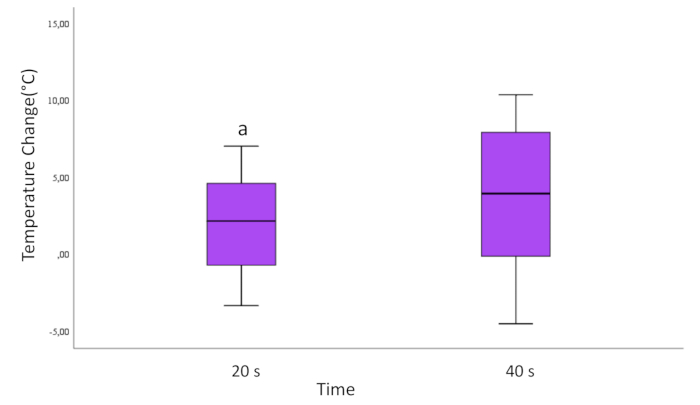
Figure 3: Temperature change according to time for all samples. The lines running up and down from the box show the minimum and maximum values of the data. The horizontal line inside the box represents the median of the data. Round marks are outliers.
a=Statistically significant difference compared with 40 s group. (p<0,05)
Please click here to view a larger version of this figure.
We analyzed the temperature change by categorizing them into two groups based on laser types (Er,Cr:YSGG and diode). The temperature change in Ti cylinders using a diode laser was observed to be greater than that in cylinders applying the Er,Cr:YSGG laser. The results are statistically significant (p=0.001; see Figure 4). In the evaluation of Ti cylinders tested only to diode laser application, the results indicated that the 40 s diode laser application yielded significantly higher outcomes compared to the 20 s application across all Watt values (p < 0.001; Figure 4). The red line within the box in the figure indicates the median value. The bars at the top and bottom indicate the maximum and minimum temperature values.

Figure 4: Temperature variation by laser types and time for all samples. The lines running up and down from the box show the minimum and maximum values of the data. The horizontal line inside the box represents the median of the data. Round marks are outliers.
a=Statistically significant difference compared with diode group. (p<0,05)
b= Statistically significant difference compared with diode laser 40 s. (p<0,05)
Please click here to view a larger version of this figure.
The latest statistical assessment of temperature change was conducted based on the Watt value. Significant differences were observed when investigating only the Watt values (p < 0.001) and Watt-time (p < 0.001) parameters in the groups that used the Er,Cr:YSGG laser. In the application of Er,Cr:YSGG laser, it was observed that the time by itself did not significantly impact temperature change (p = 0.959). Upon evaluating the temperature change in all Ti cylinders exposed to the diode laser, taking into account Watt, time, and Watt-time variables, a statistically significant difference was observed (p < 0.05). The temperature range of the diode laser groups with 1.8 Watt applied to the Ti cylinder surfaces was markedly greater than that in the diode laser groups with 0.8 Watt applied (p = 0.006; Figure 5).
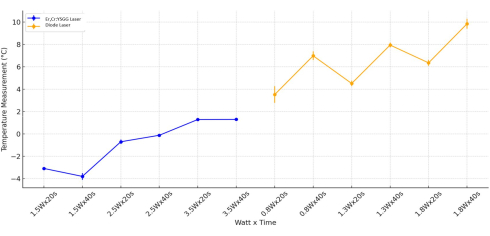
Figure 5: Combined temperature analysis. The analysis was done for Er,Cr:YSGG, and Diode Laser types by Watt and Time. Please click here to view a larger version of this figure.
Imaging analysis
In SEM images, a micron-sized porous structure was observed in all groups, which is the expected appearance of sandblasted, acid-roughened implant surfaces. At 5000x magnification, laser-treated titanium surfaces showed visible enlargement of micron-sized pores compared to the control group (red circles). At 250x and 1000x magnification, titanium surfaces treated with Er,Cr:YSGG, and diode lasers for 40 s showed more melting than those treated for 20 s (Figure 6). In AFM images, the distribution of surface indentations in the control group was more homogeneous than in the laser-treated groups (Figure 7, Figure 8). Since the AFM images only visualized a very small area of 25µm2 of the flat surface of the titanium cylinder, we could not get a detailed result about the entire surface.

Figure 6: SEM images of all study groups. The 6 diode laser groups are labeled D1-D6, while the 6 Er,Cr: YSGG laser groups are labeled E1-E6. Images are taken at 250x, 1000x, 5000x, magnification. Please click here to view a larger version of this figure.

Figure 7: AFM image of the control group. Please click here to view a larger version of this figure.

Figure 8: AFM images of all study groups. The 6 diode laser groups are labeled D1-D6 while the 6 Er,Cr: YSGG laser groups are labeled E1-E6. Please click here to view a larger version of this figure.
Surface roughness results
The roughness parameter did not show a statistically significant difference in the variables laser type (p = 0.841), Watt (p = 0.900), time (p = 0.399), and in the evaluation of laser type, Watt, and time variables together (p = 0.924; Figure 9).

Figure 9: Roughness analysis by laser type, watt, and time. Please click here to view a larger version of this figure.
Considering these results, we can conclude that Er,Cr:YSGG and diode lasers are safe for decontaminating the titanium surface in peri-implant disease. The temperature variation was below 10 °C, indicating that the parameters were within the safe range. At the same time, the profilometer value did not change significantly, indicating that there are no disadvantages in terms of surface roughness. Facial changes were detected in the imaging analysis, but this could not be supported by the roughness analysis. The results of the study support that the laser parameters used are within the safe range.
a=Statistically significant difference compared with diode group. (p<0,05)
b= Statistically significant difference compared with diode laser 40 s. (p<0,05)
Discussion
A significant discussion is underway over the optimal method for decontaminating implant surfaces in the treatment of peri-implantitis. Previous publication has proposed the utilization of local or systemic medicines, laser application, mechanical and/or chemical cleaning, and implantoplasty. Our study findings revealed that all measured temperatures rise under the critical safety threshold of 10 °C13. However, keeping in mind that this is an in vitro study and cannot always replicate clinical conditions, it was observed that the use of the Er,Cr:YSGG laser and diode laser caused melting changes and micropores in the implant topography, while the surface roughness did not change.
The use of Ho:YAG and Nd:YAG33 lasers for decontamination was reported as inappropriate due to surface effects; however, Er,Cr:YSGG lasers34 and diode lasers18 were found to be effective for this purpose. The diode laser enhances healing in surrounding tissues via HBD-2 expression stimulated by TGF-β1 signaling. The study revealed a reduction in surface roughness and P. gingivalis colonization, alongside an increase in fibroblast viability and osteoblast differentiation, following the application of the Er,Cr:YSGG laser in a zigzag motion on the titanium surface35. The results of this study showed that the Er,Cr:YSGG laser did not cause any thermal damage to titanium surfaces at energy settings up to 3.5 W up to 40 s. This finding correlates with a literature review published by Smeo et al.36, which determined that erbium lasers can exert an antibacterial impact without exceeding the critical temperature threshold when utilized with the correct laser parameters.
The 940 nm diode laser parameters in this investigation were 0.8 W, 1.3W, and 1.8 W, which included different power output and irradiation times of 20 s and 40 s13. In two different studies evaluating the use of diode lasers on titanium surfaces, 20 s37 and 40 s38 were used as the application time. Similarly, Er,Cr:YSGG lasers applied on titanium and tooth surfaces were used with application times of 20 s39 and 40 s40. In one study, a diode laser exceeded the critical temperature in 18 seconds13. In diode laser applications, it has been recommended to avoid prolonged exposure to the root surface to prevent thermal damage to the pulp (critical threshold 5.6 °C)28,41. A study evaluating the effect of the use of various lasers on the temperature change of titanium surfaces reported that Er:YAG, CO2, Nd:YAG and diode lasers did not exceed the critical temperature change of 10°C in a water tank42. Similarly, in this study, the groups of 940 nm diode lasers generated a significantly faster temperature rise; however, the final temperature values were below the critical threshold. In the application of a 940 nm diode laser, the increase in temperature can be decreased by selecting a reduced power output and minimizing the time of irradiation. These results indicate a positive relationship between increased power/energy density13,43,44 and elevated temperature in the absence of water cooling, emphasizing the significance of water cooling during irradiation like Er,Cr:YSGG laser16,45.
Mechanical and 3D-optical (contact and non-contact) profilometry are the most popular in vitro methods for quantitative measurement of dental material nano topography and implant surface roughness, while SEM images are the gold standard for qualitative evaluation31. Measuring roughness with a contact profilometer may cause damage to the surface and lead to inaccurate measurements46. While SEM imaging was unable to facilitate quantitative and qualitative analysis of samples, AFM images could provide quantitative information in terms of surface roughness and 3D depth47. Morphological alterations were noted on the implant surfaces post-laser treatment, characterized by an increase in micropore diameter, a melted morphology, and an increased prevalence of pitted micropores compared to the control group. Under these experimental conditions, the surface of the Ti cylinder during SEM analysis revealed surface alterations. Moreover, these alterations were affected by the laser type, power utilized, and time spent on laser irradiation. The authors agreed with the conclusion that levels of surface damage and time in both diode18 and Er,Cr:YSGG21,48 lasers correlate with increasing power. Further research should examine if these modifications have therapeutic implications.
Dental implant surface roughness, also known as microtopography, is a crucial factor influencing osseointegration. In a recent study, titanium surfaces were treated with four different protocols. Titanium surface and mesenchymal stem cells were preserved on the laser-treated surface and stem cell adhesion results were better than other techniques (Ti-Ni brush, Air-flow, and dental bur)49. All Ra values of the examined Ti cylinder were reduced during laser irradiation; however, no statistically significant differences were seen before and post-irradiation. Diode laser irradiation reduced surface roughness by melting the Ti surface when greater power levels were used. Those findings are consistent with a previous study by Stübinger et al.50, in which an 810 nm diode laser was used to decontaminate the implant surface and showed no significant effect on the surface while being contradictory with the results of the study conducted by Kim et al.51 and Rezeka et al.17,using 940 nm wavelength when treated with 2 and 3 W powers and observed increased surface roughness with laser application.
This study is limited by the lack of cellular and microbiological testing. The current study aims to assess the topographical changes in Ti cylinder following diode and Er,Cr:YSGG laser irradiation; nevertheless, the biological implications of the various treatments require further in vitro and in vivo investigation. Another limitation is that the statistical analysis of surface roughness conducted in this study involves only profilometer data. The AFM approach proved effective in evaluating the efficacy of two laser types frequently utilized in dentistry.
Conclusions
In our investigation, none of the temperature increases exceeded the physiological threshold of 10 °C. Consequently, statistically significant temperature differences were considered clinically irrelevant. Laser type and power did not significantly affect RA; therefore, irradiation of a 0.8, 1.3, and 1.8 W diode laser and an Er,Cr:YSGG laser with 1.5 W, 2.5 W, and 3.5 W for 20 s and 40 s can clean the Ti surface without damage. Nevertheless, these findings were conducted in vitro, and clinical trials will be needed to verify the results of this study. The current study investigated various techniques simulating a clinical scenario of implant debridement.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
The titanium cylinders used in the study were produced by Naxis Implant.
Materials
| Name | Company | Catalog Number | Comments |
| Atomic Force Microscopy | ezAFM | Compact AFM Model | |
| Diode | Biolase | Epic 10, 940 nm Wavelength | |
| E3 Tip | Fiber Diameter: 300 µm, Fiber Length: 9 mm | ||
| Er,Cr:YSGG Laser | Iplus | 2780 nm Wavelength | |
| Profilometer | Mitutoyo | Surftest SJ-201 Model | |
| RFPT-14 Tip | Outer Tip Diameter: 580 µm, Tip Length: 14 mm | ||
| Scanning Electron Microscope | FEI | Quanta FEG 250 Model | |
| Stand | Custom Design | Rhinoceros application, Flamix PLA filament, Bambulab X1C Device | |
| Thermometer | Keithley | 2000 Series Model, K tip termokulp | |
| Titanium Cylinder | Naxis | 10 mm height, 5 mm diameter, SLA Surface, Titanium |
References
- Guillaume, B. Dental implants: A review. Morphologie. 100 (331), 189-198 (2016).
- Henry, P. J. Tooth loss and implant replacement. Aust Dent J. 45 (3), 150-172 (2000).
- Berglundh, T., Armitage, G., Araujo, M. G., et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 89 (1), S313-S318 (2018).
- Herrera, D., Berglundh, T., Schwarz, F., et al. Prevention and treatment of peri-implant diseases—The EFP S3 level clinical practice guideline. J Clin Periodontol. 50 (S26), 4-76 (2023).
- Guarnieri, R., Reda, R., Di Nardo, D., Pagnoni, F., Zanza, A., Testarelli, L. Prevalence of Peri-Implant Mucositis, Peri-Implantitis and Associated Risk Indicators of Implants with and without Laser-Microgrooved Collar Surface: A Long-Term (≥20 Years) Retrospective Study. J Pers Med. 14 (4), (2024).
- Figuero, E., Graziani, F., Sanz, I., Herrera, D., Sanz, M. Management of peri‐implant mucositis and peri‐implantitis. Periodontology 2000. 66 (1), 255-273 (2014).
- Tomasi, C., Wennström, J. L. Full-mouth treatment vs. the conventional staged approach for periodontal infection control. Periodontology 2000. 51 (1), 45-62 (2009).
- Baima, G., Citterio, F., Romandini, M., et al. Surface decontamination protocols for surgical treatment of peri‐implantitis: A systematic review with meta‐analysis. Clin Oral Implants Res. 33 (11), 1069-1086 (2022).
- Subramani, K., Wismeijer, D. Decontamination of titanium implant surface and re-osseointegration to treat peri-implantitis: a literature review. Int J Oral Maxillofac Implants. , Accessed August 4, 2024 (2012).
- Świder, K., Dominiak, M., Grzech-Leśniak, K., Matys, J. Effect of different laser wavelengths on periodontopathogens in peri-implantitis: A review of in vivo studies. Microorganisms. 7 (7), 189(2019).
- Mizutani, K., Aoki, A., Coluzzi, D., Yukna, R., Wang, C. Y., Pavlic, V., Izumi, Y. Lasers in minimally invasive periodontal and peri‐implant therapy. Periodontology 2000. 71 (1), 185-212 (2016).
- Eriksson, R. A., Albrektsson, T. The effect of heat on bone regeneration: An experimental study in the rabbit using the bone growth chamber. J Oral Maxillofac Surg. 42 (11), 705-711 (1984).
- Geminiani, A., Caton, J. G., Romanos, G. E. Temperature change during non-contact diode laser irradiation of implant surfaces. Lasers Med Sci. 27 (2), 339-342 (2012).
- Alpaslan Yayli, N. Z., Talmac, A. C., Keskin Tunc, S., Akbal, D., Altindal, D., Ertugrul, A. S. Erbium, chromium-doped: yttrium, scandium, gallium, garnet and diode lasers in the treatment of peri‐implantitis: Clinical and biochemical outcomes in a randomized-controlled clinical trial. Lasers Med Sci. 37 (1), 665-674 (2022).
- Peters, N., Tawse-Smith, A., Leichter, J., Tompkins, G. Laser therapy: The future of peri-implantitis management. J Periodontol. 22 (1), 1(2012).
- Alhaidary, D., Franzen, R., Hilgers, R. D., Gutknecht, N. First investigation of dual-wavelength lasers (2780 nm Er,Cr:YSGG and 940 nm diode) on implants in a simulating peri-implantitis situation regarding temperature changes in an in vitro pocket model. Photobiomodul Photomed Laser Surg. 37 (8), 508-514 (2019).
- Rezeka, M. A., Metwally, N. A., Abd El Rehim, S. S., Khamis, M. M. Evaluation of the effect of diode laser application on the hydrophilicity, surface topography, and chemical composition of titanium dental implant surface. J Prosthodont. 2024, 1-8 (2025).
- Khalil, M. I., Sakr, H. Implant surface topography following different laser treatments: An in vitro study. Cureus. 15 (5), e38731(2023).
- Tosun, E., Tasar, F., Strauss, R., Kivanc, D. G., Ungor, C. Comparative evaluation of antimicrobial effects of Er:YAG, diode, and CO2 on titanium discs: An experimental study. J Oral Maxillofac Surg. 70 (5), 1064-1069 (2012).
- Stübinger, S., Homann, F., Etter, C., Miskiewicz, M., Wieland, M., Sader, R. Effect of Er:YAG, CO and diode laser irradiation on surface properties of zirconia endosseous dental implants. Lasers Surg Med. 40 (3), 223-228 (2008).
- Park, J., Heo, S., Koak, J., Kim, S. K., Han, C. H., Lee, J. H. Effects of laser irradiation on machined and anodized titanium disks. Int J Oral Maxillofac Implants. 27 (6), Accessed September 22, 2024 1215-1221 (2012).
- Aoki, A., Mizutani, K., Schwarz, F., et al. Periodontal and peri-implant wound healing following laser therapy. Periodontol 2000. 68 (1), 217-269 (2015).
- Roncati, M., Lucchese, A., Carinci, F. Non-surgical treatment of peri-implantitis with the adjunctive use of an 810-nm diode laser. J Indian Soc Periodontol. 17 (6), 812-817 (2013).
- Romanos, G. E., Gutknecht, N., Dieter, S., Schwarz, F., Crespi, R., Sculean, A. Laser wavelengths and oral implantology. Lasers Med Sci. 24 (6), 961-970 (2009).
- Hauser-Gerspach, I., Stübinger, S., Meyer, J. Bactericidal effects of different laser systems on bacteria adhered to dental implant surfaces: An in vitro study comparing zirconia with titanium. Clin Oral Implants Res. 21 (3), 277-283 (2010).
- Hafeez, M., Calce, L., Hong, H., Hou, W., Romanos, G. E. Thermal effects of diode laser-irradiation on titanium implants in different room temperatures in vitro. Photobiomodul Photomed Laser Surg. 40 (8), 554-558 (2022).
- Koopaie, M., Kia Darbandsari, A., Hakimiha, N., Kolahdooz, S. Er,Cr:YSGG laser surface treatment of gamma titanium aluminide: Scanning electron microscopy-energy-dispersive X-ray spectrometer analysis, wettability and Eikenella corrodens and Aggregatibacter actinomycetemcomitans bacteria count - in vitro study. Proc Inst Mech Eng H. 234 (8), 769-783 (2020).
- Kayar, N. A., Hatipoǧlu, M. Could we set a convenient irradiation time to reduce the possibility of thermal pulp damage during diode laser as an adjunct to periodontal treatment? An in vitro analysis. Photobiomodul Photomed Laser Surg. 39 (7), 480-485 (2021).
- Barrak, H., Mahdi, S. S., Alkurtas, S. A., Size, P. Clinical applications of a 940 nm diode laser for laser troughing versus conventional method: A preliminary study. Iraqi J Laser. 23 (2), (2024).
- Beer, F., Körpert, W., Passow, H., et al. Reduction of collateral thermal impact of diode laser irradiation on soft tissue due to modified application parameters. Lasers Med Sci. 27 (5), 917-921 (2012).
- Schwarz, F., Nuesry, E., Bieling, K., Herten, M., Becker, J. Influence of an erbium, chromium-doped yttrium, scandium, gallium, and garnet (Er,Cr:YSGG) laser on the reestablishment of the biocompatibility of contaminated titanium implant surfaces. J Periodontol. 77 (11), 1820-1827 (2006).
- Al-Omari, W. M., Palamara, J. E. The effect of Nd:YAG and Er,Cr:YSGG lasers on the microhardness of human dentin. Lasers Med Sci. 28 (1), 151-156 (2013).
- Kreisler, M., Götz, H., Duschner, H., d’Hoedt, B. Effect of Nd:YAG, Ho:YAG, Er:YAG, CO2, and GaAlAs laser irradiation on surface properties of endosseous dental implants. Int J Oral Maxillofac Implants. 17 (5), 202-209 (2002).
- Kottmann, L., Franzen, R., Conrads, G., Wolfart, S., Marotti, J. Effect of Er,Cr:YSGG laser with a side-firing tip on decontamination of titanium disc surface: An in vitro and in vivo study. Int J Implant Dent. 9 (1), 7(2023).
- Yao, W. L., Lin, J. C. Y., Salamanca, E., et al. Er,Cr:YSGG laser performance improves biological response on titanium surfaces. Materials. 13 (3), 756(2020).
- Smeo, K., Nasher, R., Gutknecht, N. Antibacterial effect of Er,Cr:YSGG laser in the treatment of peri-implantitis and their effect on implant surfaces: A literature review. Lasers Dent Sci. 2 (2), 63-71 (2018).
- Fletcher, P., Linden, E., Cobb, C., Zhao, D., Rubin, J., Planzos, P. Efficacy of removal of residual dental cement by laser, ultrasonic scalers, and titanium curette: An in vitro study. Compend Contin Educ Dent. , (2025).
- Lollobrigida, M., Fortunato, L., Serafini, G., et al. The prevention of implant surface alterations in the treatment of peri-implantitis: Comparison of three different mechanical and physical treatments. Int J Environ Res Public Health. 17 (8), 2624(2020).
- Arora, S., Lamba, A. K., Faraz, F., Tandon, S., Ahad, A. Evaluation of the effects of Er,Cr:YSGG laser, ultrasonic scaler and curette on root surface profile using surface analyser and scanning electron microscope: An in vitro study. J Lasers Med Sci. 7 (4), 243-249 (2016).
- Jin, S. H., Lee, E. M., Park, J. B., Kim, K. K., Ko, Y. Decontamination methods to restore the biocompatibility of contaminated titanium surfaces. J Periodontal Implant Sci. 49 (3), 193-204 (2019).
- Kayar, N. A., Hatipoǧlu, M. Can we determine an appropriate timing to avoid thermal pulp hazard during gingivectomy procedure? An in vitro study with diode laser. Photobiomodul Photomed Laser Surg. 39 (2), 94-99 (2021).
- Monzavi, A., Fekrazad, R., Chinipardaz, Z., Shahabi, S., Behruzi, R., Chiniforush, N. Effect of various laser wavelengths on temperature changes during peri-implantitis treatment: An in vitro study. Implant Dent. 27 (3), 311-316 (2018).
- Valente, N. A., Calascibetta, A., Patianna, G., Mang, T., Hatton, M., Andreana, S. Thermodynamic effects of 3 different diode lasers on an implant-bone interface: An ex-vivo study with review of the literature. J Oral Implantol. 43 (2), 94-99 (2017).
- Leja, C., Geminiani, A., Caton, J., Romanos, G. E. Thermodynamic effects of laser irradiation of implants placed in bone: An in vitro study. Lasers Med Sci. 28 (6), 1435-1440 (2013).
- Strever, J. M., Lee, J., Ealick, W., et al. Erbium, chromium:yttrium-scandium-gallium-garnet laser effectively ablates single-species biofilms on titanium disks without detectable surface damage. J Periodontol. 88 (5), 484-492 (2017).
- Bourauel, C., Fries, T., Drescher, D., Plietsch, R. Surface roughness of orthodontic wires via atomic force microscope, laser specular reflectance, and profilometry. Eur J Orthod. 20 (1), Accessed February 13, 2025 79-92 (1998).
- Choi, S., Kim, J. H., Kim, N. J., et al. Morphological investigation of various orthodontic lingual bracket slots using scanning electron microscopy and atomic force microscopy. Microsc Res Tech. 79 (12), 1193-1199 (2016).
- Huang, H. H., Chuang, Y. C., Chen, Z. H., Lee, T. L., Chen, C. C. Improving the initial biocompatibility of a titanium surface using an Er,Cr:YSGG laser-powered hydrokinetic system. Dent Mater. 23 (4), 410-414 (2007).
- Furtsev, T. V., Koshmanova, A. A., Zeer, G. M., et al. Laser cleaning improves stem cell adhesion on the dental implant surface during peri-implantitis treatment. Dent J. 11 (2), 30(2023).
- Stübinger, S., Homann, F., Etter, C., Miskiewicz, M., Wieland, M., Sader, R. Effect of Er:YAG, CO and diode laser irradiation on surface properties of zirconia endosseous dental implants. Lasers Surg Med. 40 (3), 223-228 (2008).
- Kim, H. K., Park, S. Y., Son, K., et al. Alterations in surface roughness and chemical characteristics of sandblasted and acid-etched titanium implants after irradiation with different diode lasers. Appl Sci. 10 (12), 4167(2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved