Method Article
A Do-it-yourself System for Scheduled Feeding of Laboratory Rodents in Their Home Cage
* These authors contributed equally
In This Article
Summary
This paper details the design, assembly, and protocol for an automated feeding system along with an alternative lid and cage modification that can be implemented on standard rodent cages with minimal modifications for tethered optogenetic or fiber photometry experiments. The feeding system offers a cost-effective tool for timed feeding and/or caloric restriction.
Abstract
Intermittent fasting and research surrounding the influence of timed caloric restriction on body weight regulation and aging outcomes are trending, both in society and in the laboratory. To study time-restricted feeding and/or caloric restriction in the laboratory, allotted quantities of food are dispersed at scheduled time intervals. Current technology for scheduled feeding of rodents involves either specialized cages with gated food entry ports, time-locked digital hoppers, or manual food delivery. Specialized equipment for such experiments can be costly and manual feeding often requires investigators to come in for late night/early morning time points, precluding prolonged studies. The automated feeding system described here provides a cost-effective tool for timed feeding and/or caloric restriction that can be implemented on standard rodent cages with minimal modifications. The protocol uses an off-the-shelf autofeeder that is outfitted to a standard microisolator cage lid (mouse, hamster, rat, or guinea pig cage lids) and can administer the desired food allotments at programmed time intervals in the animal's home cage. Our design can be modified to accept various feeders or slight variations in cage dimensions. We provide the design, assembly, and protocol for the feeding system along with an alternative lid and cage modification for tethered optogenetic or fiber photometry experiments.
Introduction
Intermittent fasting is an important area of metabolic and aging research. Proper alignment of feeding and circadian rhythm can alter the effect of diet on body weight1,2,3,4. Further, prolonged intermittent fasting is associated with increased longevity and improved age-related disorders5,6,7,8. Research studies implementing long-term fasting protocols (>20 days) typically require specialized equipment. While it is possible to deliver food manually for scheduled feeding protocols, this may require an investigator to be present at numerous off-hour time points for an extended period. For instance, three feeding times that are 4 h apart would require manual delivery at 8:00 pm, 12:00 am, and 4:00 am; likely a compliance challenge to sustain for more than a few days.
Alternatives to manual food delivery for laboratory rodents are 1) specialized cages with automated gate access to food or 2) digital hopper systems that provide automated and programmed access to food, such as FED39. Caging systems with gated food access are costly and some do not provide a suitable home-cage environment for long-term studies. For example, traditional operant conditioning boxes are capable of automated food delivery but have a wire grated floor that is not conducive for long-term housing. The BioDAQ system from Research Diets provides food and water via gated external ports. Programable automated gates allow for long-term scheduled feeding or drinking studies, however, the system is a costly investment. Mechanized hopper systems are yet another tool for automated scheduled feeding protocols9,10. The two cited mechanized hoppers come with the caveat of reducing the home cage living space. Food-restricted hungry animals may chew on the feeding apparatuses and/or cage debris may interfere with the hopper mechanics for long-term studies. Finally, a mechanized hopper developed by the Takahashi research group along with Phenome Technology was specifically designed for long-term scheduled feeding studies3. In this system, a mechanized hopper is positioned above the wire rack of a standard cage with a chute that delivers food. The Phenome system, however, does not provide flexibility for animals tethered to a patch cable (optogenetics/fiber photometry experiments), and it is relatively expensive compared to the autofeeder apparatus described here.
In response to the prevailing challenges in scheduled feeding protocols, we propose a cost-effective remedy. Our automated system facilitates precise food delivery at predetermined intervals. With a combination of 3D printed parts along with a commercially available automatic fish feeder, we devise a do-it-yourself (DIY) external feeding system that can be outfitted on stand-alone rodent cages that use microisolator tops. The feeding system and parts described here can be directly applied to any of the following rodent cages: mouse, rat, hamster, or guinea pig, provided that the stand-alone cages use microisolator tops. Because the cages vary in size, placements may vary slightly, but the 3D-printed brackets, feeding system, and external water bottle design are generally applicable across stand-alone rodent cages with microisolator lids.
We profile one example autofeeder, but many other off-the-shelf autofeeders are available and can be fitted to the modular system with slight changes to the 3D printed parts. To facilitate ease of adjusting the 3D printed holder to alternative feeders or slight variations in caging dimensions, we prove a modifiable, 3-dimensional SolidWorks file with a companion STL file.
The external placement of the autofeeder provides full space within the home-cage and allows for detachment, sterilization, and reloading or replacement of the feeder without disruption to the animal. The automated feeding system is efficient for long-term recordings, affordable, and amenable to various time-restricted feeding and/or caloric restriction protocols. We provide detailed technical drawings of the cage assemblies, design, and protocol for implementing the feeding system, along with sample data. Second, we provide a set of instructions to modify standard caging for the use of the autofeeder with animals that are tethered to a patch cable for optogenetic or fiber photometry experiments.
Protocol
All procedures were approved by the University of Massachusetts Amherst Institutional Animal Care and Use Committee.
1. Assembly of autofeeder onto a standard microisolator cage lid (Figure 1)
- Using a rotary tool with a 1.5" cutting wheel, cut out a 20 x 25 mm square into a microisolator cage lid. Position the opening 2.75 cm from the edge of the lid and centered above the wire rack (Figure 1B, left).
- 3D print a bracket to couple the autofeeder onto the cage lid (Figure 1A and Supplemental File 1). Snap the bracket onto the hole of the cage lid (step 1.1; Figure 1B, center) and slide the autofeeder onto the top of the secured bracket (Figure 1B [right] ,C).

Figure 1: Autofeeder bracket and cage lid modification and assembly. (A) Technical drawing of the autofeeder bracket (see SolidWorks/STL files in Supplemental File 1 for scale in 3D). (B) From left to right, cage-top with 20 x 25 mm hole; bracket installed on cage top and mounted with Petbank autofeeder. (C) Assembled Petbank Autofeeder on cage with food pellet dropped onto a standard wire rack with water bottle. Scale bars = 25 cm). Please click here to view a larger version of this figure.
2. Programming and usage of the example off-the-shelf autofeeder
- For timed experiments, set feeding times first and synchronized feeder clock last as subsequent adjustments will shift clock time.
- Ensure the feeder is sufficiently charged. Press and hold the Settings button until the hour setting on the screen is blinking.
- Continue to the time setting. Using the up and down arrows, adjust the time to the first desired scheduled feeding period.
- Set the number of allotments for this time point (indicated by the number of dots next to the feed setting). Repeat steps 2.2-2.4, for up to two more time points.
- Load the autofeeder chambers with the desired food allotments of ≤1.5 g of standard rodent chow. If more food is needed, set the feeder to deliver multiple allotments at a given time point. The example feeder has 16 chambers.
NOTE: Food can be ordered to size or trimmed with scissors. - Label one chamber with tape or paint to confirm programmed chamber rotation and food delivery. Check daily while running the feeders.
3. Modified caging system for tethered animals with the autofeeder
- Optional replacement lid insert for animals tethered to a patch cord (Figure 2)
- Laser cut a piece of 3 mm-thick, clear acrylic to the specifications of Figure 2A (see also Supplemental File 2). The acrylic insert is designed with tabs to snap into the cage top. The center slit (190 x 5 mm) allows passage of the patch cable. The 20 x 25 mm square hole fits the autofeeder bracket; 20 ventilation holes allow for fresh air circulation.
- Using either a flat head screwdriver or wedge tool, pry off the perforated cage top cover of a standard microisolator lid. Work to release the tabs from the tab slots and remove both the perforated insert and the filter paper (Figure 2C, left and center).
- Under the perforated top and filter paper is an internal ribbed support grid. Use a rotary tool and 1.5 inch cutting wheel to remove the support grid; following the arrow indicators shown in the center of Figure 2C. After cutting to remove the support grid, file to smooth the cut edge.
- Snap the replacement acrylic plate insert (step 3.1) into the cage top. Start installation by sliding the plate tabs into the tab slots along either of the short edges and then one of the long edges and gently press until the plate snaps into place (Figure 2C [right] ,D).
- Optional external water bottle bracket for animals tethered to a patch cord (Figure 3)
- Drill a port for the water bottle sipper: 11 mm diameter hole, 50 mm from the floor, centered on the short edge of the cage (Figure 3B and Supplemental File 3).
- On either side of the center hole (± 27.5 mm), drill two 6 mm diameter holes. Optionally, use an 8 mm square punch tool to bore square holes (shown in Figure 3B).
- 3D print an external water bottle bracket (Figure 3A and Supplemental File 4). Mount the water bottle bracket to the cage bottom with carriage bolts (2 cm long, 6 x 1 mm, or 0.75" long 1/4 x 20; Figure 2B,C).
- For a water bottle, fit a standard 50 mL conical tube, capped with a sipper/stopper assembly (#7 Stopper W/ 3-1/2" curved sipper, see Table of Materials) on the external bracket. Refill water every 2-3 days.

Figure 2: Assembly of acrylic plate onto the cage top. (A,B) Technical drawings of acrylic plate and feeder port plug. (C) Left, standard cage top with perforated insert and filter. Center, removal of perforated insert and filter (ribbed grid must be cut out along the arrow-indicated lines). Right, acrylic plate snapped into place. (D) Complete lid assembly. Scale bars = 25 cm. Please click here to view a larger version of this figure.
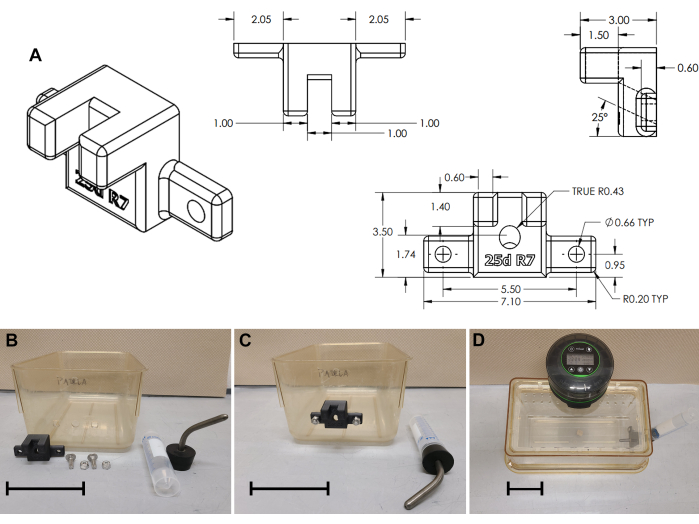
Figure 3: Water bottle bracket assembly. (A) Technical drawing of 3D printed water bottle bracket. (B) Cage modifications and water bottle bracket assembly; (C) Bracket installed; (D) Complete cage assembly. Scale bars = 10 cm. Please click here to view a larger version of this figure.
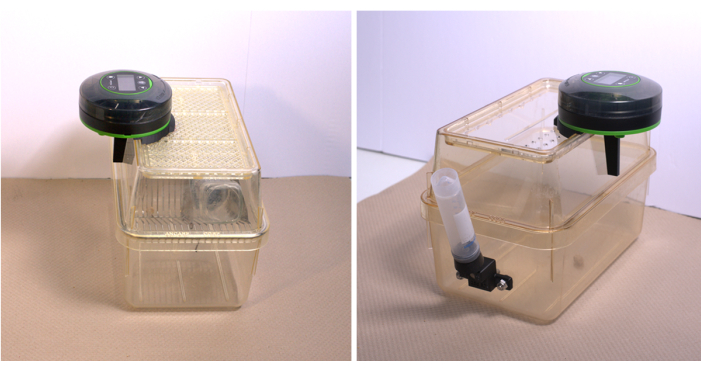
Figure 4: Summary of complete cage assembly with the autofeeder. (A) Standard cage with wire rack, (B) modified cage for tethered animals. Please click here to view a larger version of this figure.
Results
A caloric restriction study (21 days) to induce infertility in female mice (autofeeder and cage design, Figure 4A)
Chronic energy deficiency is sufficient to inhibit fertility in mammals, a response that is conserved in rodents and humans alike11,12. In this study, we use caloric restriction to induce long-term bodyweight loss. Adult female C57BL/6 mice, 15-18 weeks old were single-housed and on a 12:12 h light:dark cycle, with lights out at Zeitgeber Time 12 (ZT12). The wire rack containing food was weighed daily for each animal over a week's interval to establish baseline food intake, 3.80 ± 0.64 g/day (mean ± SE). Next, food was restricted to induce weight loss. Food was rationed into three portions and distributed by the autofeeder over three intervals: ZT 12, 16, and 20 (cage configuration, Figure 4A). For the initial 48 h, the animals were restricted to 50% of the baseline food intake. Then, the animals were restricted to ~70% of the baseline food over the next 19 days, with slight adjustments in the latter half of the restriction to maintain 80-90% of the baseline weight (Figure 5A, bottom).
Between ZT 0 and ZT 1, the animals were weighed and assessed to stage the ovulatory cycle by histological characterization of cells gathered by a vaginal lavage with 0.1 M phosphate-buffered saline. The cycle length was monitored for at least 11 days before and during 80-90% weight loss. Sustained weight loss of greater than 10% was sufficient to induce infertility in female mice, as perviously published11.
The autofeeders were surveyed daily for proper rotation and food delivery (protocol step 3.1). Any food left on the ground or remaining in the wire rack was discarded. The example feeder profiled here has 16 chambers and can be set to deliver food at three intervals (see Table of Materials). We determined that the maximum volume of chow that fits in a chamber is 2 g, which can be cut or ordered to size. We also determined that food delivery takes ~10 s once the programmed time is reached. It takes 7 s for the floor gate to fully open and then another 3 s for the chamber to move over the opening, dispensing the food pellet. There are noises that coincide with gate opening and the pellet of food dropping into an empty wire rack (Video 1, with sound recording).
Over the course of 21 days, we observed complete accuracy of rotations and food delivery using this specific feeder from Petbank (Table of Materials). At the time of testing, the feeders were between 3 and 6 months from their initial use and the internal rechargeable batteries maintained full charge indications for the entirety of the 21 day trial. Because this was a relatively small sample of feeders (n = 4), we subsequently conducted another 21 day study with 10 autofeeders, set to distribute food at a matched rate. The subsequent study was performed 10 months after the initial use of the feeders. Over the 21 day trial, one feeder lost a single bar of power (~25% power loss) at day 15 and another lost a single bar at day 17, while the remaining 8 feeders had full charge indications for the entire 21 days. The power level on the example feeder is indicated as four bars. Our quantification assumes that each bar represents 25% power. As with our initial test, the feeders in the subsequent study again performed rotation and food delivery without error for the duration of the study.

Figure 5: Bodyweight trajectory with overlayed estrous cycling data in female mice during a caloric restriction using the autofeeder. (A) Average weight loss trajectory (bottom) using the autofeeder to deliver 50-70% of baseline food intake over three time points (4 h apart, starting at lights out). Representative estrous cycle data aligned with weight loss (top). Grey highlight indicates weight loss greater than 10% of starting weight. Arrow indicates return to ad libitum food access by filling the wire rack with food and removing the autofeeder. (B) Estrous cycling during weight loss; within-subject comparison of % time in diestrus when animals are between 1% and 10% (open bar) weight loss versus >10% weight loss (grey bar; note: arresting in diestrus is a sign of infertility) n = 4. (C) Waterfall plot of weight loss versus cycling. With up to 8% weight loss, 100% of the animals were fertile and cycling, while at 20% weight loss, 100% of the animals were acyclic and infertile. Abbreviations: P = proestrus; E = estrus; D = diestrus. Error bars represent the standard error of the mean Please click here to view a larger version of this figure.
Video 1: Noises indicating gate opening and the pellet of food dropping into an empty wire rack. Please click here to download this Video.
Supplemental File 1: Zip file containing the technical drawing and SolidWorks + STL files for the autofeeder bracket. Please click here to download this File.
Supplemental File 2: Technical drawing of the acrylic plate. Please click here to download this File.
Supplemental File 3: Zip file containing the technical drawing and SolidWorks + STL files for the port plug. Please click here to download this File.
Supplemental File 4: Zip file containing the technical drawing, SolidWorks + STL files for the water bottle bracket and bottle holder. Please click here to download this File.
Discussion
In preclinical animal models, caloric restriction is a powerful experimental tool to precisely manipulate and study metabolism. Sustained weight loss has implications in obesity, diabetes, aging, fertility, and menopause (a type of reproductive aging), all of which are important translational endpoints. Caloric restriction paradigms, however, can be difficult to sustain for an extended time. In this manuscript, we offer a cost-effective solution to achieve long-term caloric restriction in a laboratory setting. Provided are instructions to couple an off-the-shelf automatic feeder to a rodent cage with a standard microisolator lid. The feeder is assembled on the animal's home cage and is programmed to deliver food at scheduled times. Scheduled food delivery controls the quantity and timing of food access and is free of a handler entering the room. Further, the cost-effective system can run for an extended period (months), improving the ability to achieve long-term caloric restriction in a laboratory setting.
Recent advances in neuroscience allow investigators to either manipulate or record from neurons that promote feeding in awake, behaving animals. Specifically, fiber photometry recordings (an in vivo recording technique) of feeding neurons have been performed with a sequence of fasting, to promote neural activity, and then refeeding, to investigate change in neural activity13,14,15. While it is best to perform in vivo recordings in the animal's home cage16, they are often conducted in an external arena to provide mobility while tethered to a patch cable. Moreover, in vivo recordings can be influenced by a handler entering the room without a means of gated food access. To address both confounding issues for photometry recordings of neurons in the feeding circuits, we designed a home-cage modification to house tethered animals for an extended period, which is coupled to the autofeeder (protocol sections 3 and 4). A limitation to this approach is that the cage lid insert design requires a laser cutter. While a rotary tool and scoring device could be used to cut the acrylic insert, the tools would likely fail at generating small tabs and a thin slit.
There are limitations to the feeding system described here. Namely, the assembly requires 3D printed parts. Further, the clock of the example autofeeder runs on a 24 h cycle, so the timing of food delivery for circadian studies must be manually aligned to the free-running period of the animal17. Finally, the device is limited to 16 chambers and three programmed time intervals. The feeder distributes food; it does not monitor consumption. Food that is not consumed over a set time, can be retrieved from the cage floor or wire rack, but there may be errors in accounting for and retrieving uneaten food.
To highlight the advantages of a commercial off-the-shelf autofeeder outfitted to a standard rodent cage, we show that the device is user-friendly and delivers precise allotments of food at specific time intervals. This system does not obstruct the home-cage area. Because the feeder is positioned outside the animal's cage and can be easily detached from the 3D-printed holder, regular maintenance, food refills, and cleaning can be performed with minimal disturbance to the animal.
As an example, we used the autofeeder to induce caloric restriction and, ultimately, infertility. We demonstrate the use of the autofeeder in a 21 day caloric restriction study (Figure 5) and have subsequently used the feeders for numerous experiments in the laboratory. As described in the results, the feeders were reliable and accurately delivered food. The internal, rechargeable battery appears to diminish somewhat over time, but after nearly a year of using the feeders, they run for a minimum of 15 days before registering even a slight power loss. It is estimated by the manufacturer that the battery can run for 60 days, starting from a full charge. For prolonged studies (>30 days), it is advisable to have fully charged replacement feeders to rotate in when the battery life of the feeder is below 50% of maximum charge.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
Jason Lê is supported by the National Institutes of Health-funded post-baccalaureate research education program.
Materials
| Name | Company | Catalog Number | Comments |
| #7 Stopper W/ 3-1/2" curved sipper tube no ball | Labex | 2067 | |
| AN75 mouse cage bottom | Ancare | AN75HT | |
| Automatic Fish Feeder for Aquarium | Petbank-tek.com | Amazon distribution (#B0BRCWP16K) | |
| Rodent Micro-filter Tops | Ancare | N10MBTPLF | |
| Rubber Stoppers, One Hole | United Scientific | S24009 | |
| standard rodent chow | Prolab IsoPro 3000 |
References
- Arble, D. M., Bass, J., Laposky, A. D., Vitaterna, M. H., Turek, F. W. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring). 17 (11), 2100-2102 (2009).
- Hatori, M., et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 15 (6), 848-860 (2012).
- Acosta-Rodriguez, V. A., de Groot, M. H. M., Rijo-Ferreira, F., Green, C. B., Takahashi, J. S. Mice under caloric restriction self-impose a temporal restriction of food intake as revealed by an automated feeder system. Cell Metab. 26 (1), 267-277.e2 (2017).
- Gallop, M. R., Tobin, S. Y., Chaix, A. Finding balance: understanding the energetics of time-restricted feeding in mice. Obesity (Silver Spring). 31 (Suppl 1), 22-39 (2023).
- Hua, L., et al. Time-restricted feeding improves the reproductive function of female mice via liver fibroblast growth factor 21. Clin Transl Med. 10 (6), e195(2020).
- Strilbytska, O., Klishch, S., Storey, K. B., Koliada, A., Lushchak, O. Intermittent fasting and longevity: From animal models to implication for humans. Ageing Res Rev. 96, 102274(2024).
- Di Francesco, A., Di Germanio, C., Bernier, M., de Cabo, R. A time to fast. Science. 362 (6416), 770-775 (2018).
- Longo, V. D., Panda, S. Fasting, Circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 23 (6), 1048-1059 (2016).
- Matikainen-Ankney, B. A., et al. An open-source device for measuring food intake and operant behavior in rodent home-cages. Elife. 10, e66173(2021).
- Sahasrabudhe, A., Guy, C. R., Greenwell, B. J., Menet, J. S. Manipulation of rhythmic food intake in mice using a custom-made feeding system. J Vis Exp. (190), (2022).
- Kreisman, M. J., Tadrousse, K. S., McCosh, R. B., Breen, K. M. Neuroendocrine basis for disrupted ovarian cyclicity in female mice during chronic undernutrition. Endocrinology. 162 (8), ebqab103(2021).
- Allaway, H. C., Southmayd, E. A., De Souza, M. J. The physiology of functional hypothalamic amenorrhea associated with energy deficiency in exercising women and in women with anorexia nervosa. Horm Mol Biol Clin Investig. 25 (2), 91-119 (2016).
- Betley, J. N., et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 521 (7551), 180-185 (2015).
- Chen, Y., Lin, Y. C., Kuo, T. W., Knight, Z. A. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell. 160 (5), 829-841 (2015).
- Mandelblat-Cerf, Y., et al. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. Elife. 4, e07122(2015).
- Kahnau, P., et al. A systematic review of the development and application of home cage monitoring in laboratory mice and rats. BMC Biol. 21 (1), 256(2023).
- Eckel-Mahan, K., Sassone-Corsi, P. Phenotyping circadian rhythms in mice. Curr Protoc Mouse Biol. 5 (3), 271-281 (2015).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved