Method Article
PET/CT With [68Ga]-NOTA-FAP-2286 for Imaging of Tendon Injuries in Rat Achilles Tendon Injury Models
* These authors contributed equally
In This Article
Summary
Here, we present a protocol to utilize [68Ga]-NOTAFAP-2286 PET/CT imaging to exhibit high sensitivity in detecting and precisely localizing tendon injuries.
Abstract
Tendon injuries, particularly those affecting the Achilles tendon, are common sports-related conditions that significantly impact patients' quality of life. Although existing diagnostic methods such as MRI and ultrasound are widely used, they may occasionally fail to provide accurate pathological information. This study aimed to investigate the feasibility of using [68Ga]-NOTA-FAP-2286 positron emission tomography/computed tomography (PET/CT) imaging for assessing an experimental rat model of Achilles tendon injury. The application of [68Ga]-NOTA-FAP-2286 PET/CT imaging demonstrated high sensitivity in detecting and precisely localizing tendon injuries, offering more comprehensive pathological insights compared to conventional imaging techniques. Fibroblast activation protein (FAP) ,a marker for activated fibroblasts, plays a crucial role in the tendon repair process. Tracer uptake started on the injured/ruptured side from the first week post modeling and peaked during the second week. These results show that FAP expression, activated by the Achilles tendon injury and rupture, reached its highest level during the second week of the repair process. By leveraging the specificity of the [68Ga]-NOTA-FAP-2286 tracer, this novel imaging approach accurately visualizes the extent and progression of tendon injuries in the animal model.
Introduction
Fibroblasts are ubiquitously distributed across nearly all tissues and typically reside in a quiescent state. Upon disruption of tissue integrity, fibroblasts become activated and migrate to the injury site, orchestrating the repair process1. Once the repair is complete, fibroblasts generally revert to their quiescent state; however, in conditions of chronic inflammation or fibrosis, they may remain persistently activated. FAP is a transmembrane protein expressed on the surfaces of activated fibroblasts. Recent studies have demonstrated a highly promising non-invasive method for tracking FAP to identify various important tumor entities, including breast, lung, and colorectal cancers2. This approach could be explored further for its potential in diagnosing tendon injuries.
Tendon injuries constitute a significant musculoskeletal issue, comprising approximately 30% of all musculoskeletal-related consultations in general medical practice3. These injuries are prevalent across various age groups and demographics, with a notably higher incidence among individuals aged 30 years or older, occupational groups engaged in repetitive motions, and athletes. Notable examples include rotator cuff injuries, Achilles tendon ruptures, patellar tendinopathy, and tennis elbow, each exhibiting distinct incidence rates and affected populations4,5,6,7. Diagnostic modalities, such as B-ultrasound and MRI, are commonly employed for assessing tendon injuries. However, given the characteristic healing process of tendons involving fibroblast accumulation around the wound surface, a study was conducted to investigate the use of Al[18F]-NOTA-FAPI-04 for Imaging tendon Injury models8. The findings support the hypothesis that PET-CT imaging with FAPI may serve as an effective method for monitoring tendon healing progress and evaluating injury severity.
[68Ga]-NOTAFAP-2286 exbibits the advantage of specifically targeting FAP in the tumor microenvironment with a prolonged retention time. It is currently used for tumor imaging. To our knowledge, PET/CT with [68Ga]-NOTA-FAP-2286 has not been used for imaging tendon injuries. Therefore, we conducted this study to explore the application of PET/CT with [68Ga]-NOTA-FAP-2286 in imaging tendon injuries.
Protocol
All animal experiments were performed in accordance with the ethical standards for animal experimentation by the First Affiliated Hospital of Zhejiang University School of Medicine. (Reference Number: 20241008). 68Ga is a radioactive element that emits positrons from decay, which quickly combine with the surrounding electrons to release gamma rays. Gamma rays can penetrate skin, posing a risk of radiation damage to the body. All experimental personnel must undergo radiation safety and protection training before conducting related experiments. During the experiments, it is necessary to wear radiation dosimeters, shielding instruments, and other protective equipment. The radioactive waste produced in the experiment needs to be appropriately disposed of after the experiment.
1. Preparation and modeling process of animal models
- Preparation
- Obtain male Sprague-Dawley (SD) rats (6-8 weeks old, ~250 g ,n = 8). Acclimate them under standard laboratory conditions with ad libitum access to food and water for 7 days. Use them to establish the models of Achilles tendon injury and rupture.
- Randomly divide the rats into two groups: group one: Achilles tendon injury model (n = 4); group two: Achilles tendon injury model (n = 4).
- Modeling process
- Prepare the instruments: scalpel, hemostatic forceps, standard forceps, isoflurane, oxygen, alcohol swabs, iodophor, fixation plate, surgical sutures, and needles.
- Anesthetize the rat in an induction chamber with an isoflurane-oxygen mixture (1:1). Secure the unconscious rat onto a fixation board and maintain anesthesia using a gas mask delivering a continuous supply of isoflurane-oxygen.
- Sterilize surgical instruments with alcohol and disinfect the surgical site.
- Establish the Achilles tendon injury model.
- Apply depilatory cream evenly to the right hind limb, wait for 5 min, and remove hair using a razor.
- Use a scalpel to make a longitudinal incision to expose the Achilles tendon.
- Compress the tendon using hemostatic forceps until it is flattened.
- Close the incision with sutures.
- Establish the model of Achilles tendon rupture.
- Follow steps 1.2.4.1 and 1.2.4.2 to expose the Achilies tendon.
- Grasp the Achilles tendon using hemostatic forceps and create a full-thickness midline incision using surgical scissors (Figure 1).
- Suture the severed tendon and close the skin incision.
- Monitor the rats for signs of distress and ensure proper wound healing. Perform PET imaging 1 week post surgery.
2. Synthesis of 68 Ga-NOTA-FAP2286
NOTE: 68Ga (half-life: 68 min) is obtained from a 67Ge/68Ga generator.
- Prepare 50 µg of the precursor, dissolve it in 800 µL of anhydrous acetonitrile, and add 3 mL of 0.1 M sodium acetate buffer solution to adjust the pH to ~4. Elute the 67Ge/68Ga generator (5 mL/3 min; make sure there are no air bubbles in the syringe).
- Draw 5 mL of 0.1 M hydrochloric acid using a silica gel-headed syringe and inject the hydrochloric into the 67Ge/68Ga generator to replace the 68Ga. Elute 68Ga (∼17 mci) into a 10 mL glass vial
NOTE: Avoid contact of the hydrochloric acid with metal. - Mix the dissolved precursor (NOTA-FAP-2286, CAS Registry Number: 2583823-71-4) and eluted 68Ga and place them in a heater at 35 °C for 20 min (the labeling reaction is shown in Figure 2).
- To purify the product, preactivate the C18 column with 10 mL of ethanol and 10 mL of water. Place the product on the C18 column and rinse it with 1 mL of 50% ethanol to obtain the final product.
- Evaporate the ethanol. Heat the final product bottle at 80 °C for ~20 min to remove excess ethanol from the solution by evaporation.
- Perform a quality control check using HPLC: Mobile phase: A (0.1% H3PO4 Aqueous solution), 72%-52%, 0-20 min; B (CH3CN), 28%-48%, 0-20 min; Flow rate: 3 mL/min.
3. Small animal PET imaging
- Secure the rat in a restrainer. Administer 500 µci of 68GA-NOTA-FAP2286 via the tail vein into both groups' rats.
- Induce anesthesia using isoflurane in an induction chamber.
- Position the fully anesthetized rat in the scanning field with the Achilles tendon at the center of the scanning field (Figure 3).
- Perform small animal PET scanning and obtain Imaging results.
- Turn on the computer connected to the small animal PET system. Click on CT Acquisition to initiate the preheating process of the CT system.
- Once the preheating is complete, select the scanning protocol and open the corresponding scanning workflow (the scan duration is preset to 10 min).
- Click on Scout View to confirm the positioning of the Achilles tendon in the center of the scanning field. If the position is correct, click Start Workflow to perform the PET and CT scans.
- Use the following parameters : NCT bed view:0-36.2-76.4 mm, CT scan time:60 s/one bed. PET: Acquire by time: 600 s; 511 KeV Photopeak Energy Level; 350 Kev Lower-Level Discrimination; 650 KeV Upper-Level Discrimination; 3.432 ns Timing Window.
- After the scan is completed, click on Reconstruction to reconstruct the scan files and obtain the imaging results. Use the Research Workplace for postprocessing of the images.
Results
We successfully synthesized [68Ga]-NOTA-FAP-2286 with a yield of more than 70% (Decay corrected), achieving a radiochemical purity exceeding 95%. The HPLC profile is shown in Figure 4. Tendon injuries models were established surgically, and [68Ga]-NOTA-FAP-2286 was administered intravenously, followed by successful small-animal PET imaging. The results are presented in the Figure 5, Figure 6, and Figure 7. As illustrated in the PET image (Figure 5), tracer uptake began on the injured/ruptured side at 30 min post injection and continued to increase over the next 90 min, showing a significant difference compared to the normal Achilles tendon and muscle tissue. In contrast, Figure 6 demonstrates that distinguishing between the injured/ruptured Achilles tendon and the normal Achilles tendon in CT images is challenging. The maximum intensity projection (MIP) image in Figure 7 shows that tracer uptake began on the injured/broken side from the first week post modeling and peaked during the second week, indicating that FAP expression, activated by the Achilles tendon injury and rupture, reaches its highest level during the second week of the repair process. Specific SUV values are provided in Table 1 and Table 2. Table 1 indicates that at 30, 60, and 90 min post injection, the maximum and mean SUV values on the fracture/injury side were significantly higher than those of the normal Achilles tendon and muscle tissue, with statistical significance observed at all three time points. Table 2 further confirms that during the initial 3 weeks, the SUV values on the fracture/injury side differed from those of the normal side and muscle tissue, with the most pronounced difference occurring in the second week. The data in Table 1 and Table 2 corroborate the imaging findings depicted in Figure 5 and Figure 7.
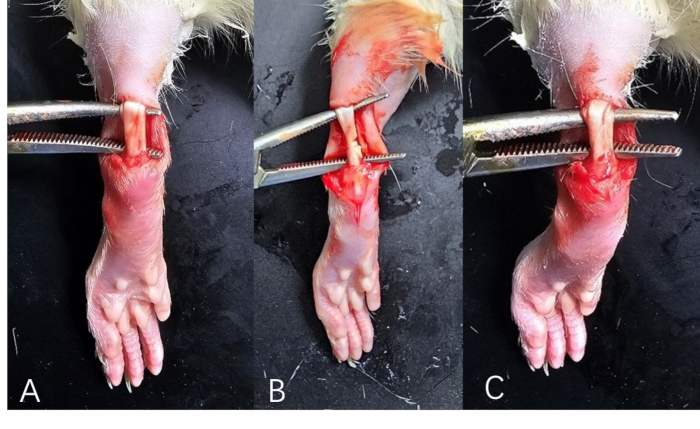
Figure 1: Rat's Achilles tendon models. (A) Achilles tendon injury model; (B) Achilles tendon rupture model. (C) Normal structure of the Achilles tendon in rats. Please click here to view a larger version of this figure.
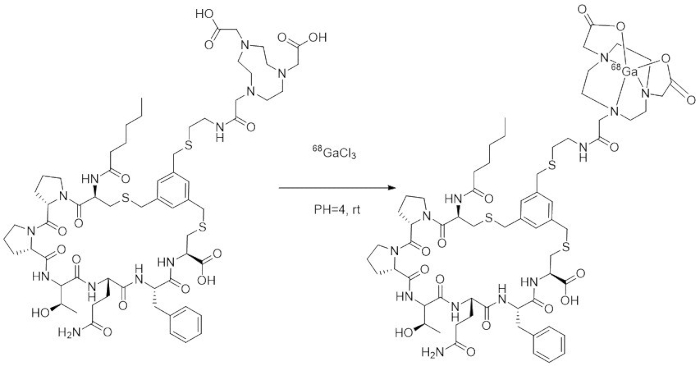
Figure 2: The labeling reaction. Please click here to view a larger version of this figure.
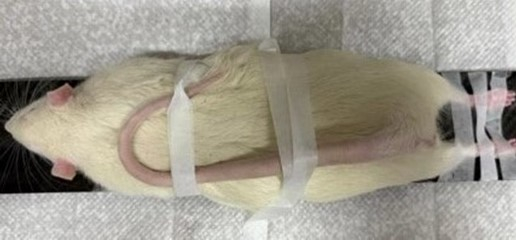
Figure 3: The special fixation method to keep the Achilles tendon of rat in the center of the scanning field. Please click here to view a larger version of this figure.
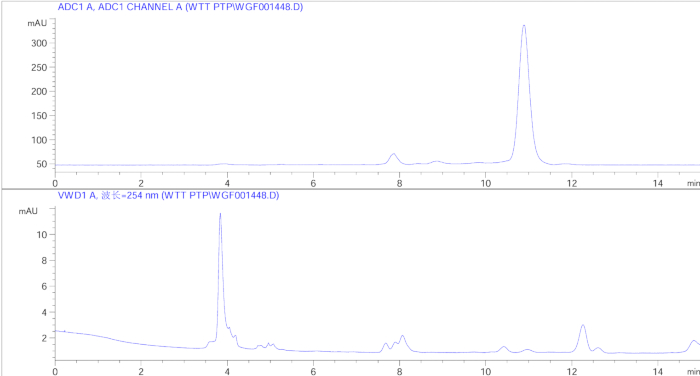
Figure 4: The radioactive HPLC and ultraviolet spectra of [68Ga]-NOTA-FAP-2286. Please click here to view a larger version of this figure.
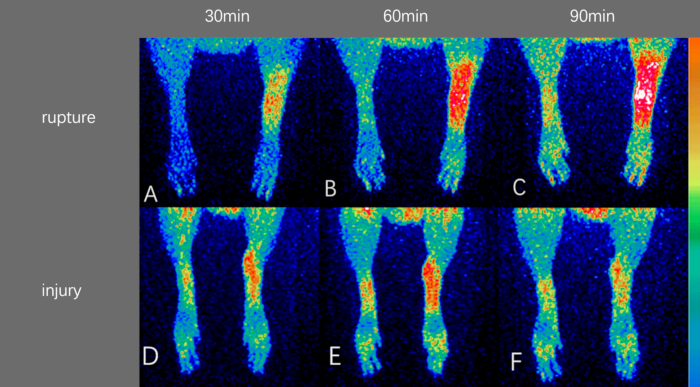
Figure 5: Results of PET imaging. Imaging Achilles tendon rupture model at (A) 30 min after injection, (B) 60 min after injection, (C) 90 min after injection. Imaging Achilles tendon injury model at (D) 30 min after injection, (E) 60 min after injection, (F) 90 min after injection. Please click here to view a larger version of this figure.

Figure 6: Results of CT imaging. CT imaging of ruptured Achilles tendon after (A) 7 days, (B) 14 days, (C) 21 days. CT imaging of injured Achilles tendon after (D) 7 days, (E) 14 days, (F) 21 days. Please click here to view a larger version of this figure.

Figure 7: Results of MIP of PET-CT Imaging. MIP of PET-CT imaging of ruptured Achilles tendon after (A) 7 days, (B) 14 days, (C) 21 days. MIP of PET-CT imaging of injured Achilles tendon after (D) 7 days, (E) 14 days, (F) 21 days. Abbreviation: MIP = maximum intensity projection. Please click here to view a larger version of this figure.
| Group1 (injury) | |||||
| Injury Achilles Tendon | Normal Achilles Tendon | Thigh muscle | Ratio | Ratio | |
| (injury/normal) | (injury/muscle) | ||||
| 30 min | |||||
| l SUVmax | 0.52±0.47 | 0.38±0.09 | 0.26±0.10 | 1.36±0.39 | 1.97±0.71 |
| l SUVmean | 0.18±0.07 | 0.11±0.08 | 0.09±0.07 | 1.63±0.51 | 1.96±0.61 |
| 60 min | |||||
| l SUVmax | 0.52±0.32 | 0.38±0.24 | 0.26±0.09 | 1.36±0.67 | 1.97±0.41 |
| l SUVmean | 0.18±0.12 | 0.13±0.08 | 0.09±0.05 | 1.38±0.55 | 2.00±0.52 |
| 90 min | |||||
| l SUVmax | 0.59±0.41 | 0.39±0.30 | 0.33±0.14 | 1.50±0.04 | 1.77±0.05 |
| l SUVmean | 0.18±0.07 | 0.09±0.03 | 0.08±0.03 | 1.98±0.90 | 2.25±1.06 |
| Group2 (rupture) | |||||
| Rupture Achilles Tendon | Normal Achilles Tendon | Thigh muscle | Ratio | Ratio | |
| (rupture/normal) | (rupture/muscle) | ||||
| 30 min | |||||
| l SUVmax | 0.52±0.12 | 0.33±0.16 | 0.26±0.09 | 1.56±0.39 | 1.99±0.50 |
| l SUVmean | 0.12±0.08 | 0.05±0.04 | 0.04±0.02 | 2.34±1.05 | 2.72±0.90 |
| 60 min | |||||
| l SUVmax | 0.73±0.60 | 0.40±0.14 | 0.49±0.31 | 1.85±0.71 | 1.49±0.20 |
| l SUVmean | 0.25±0.19 | 0.09±0.06 | 0.07±0.06 | 2.85±0.31 | 3.53±0.11 |
| 90 min | |||||
| l SUVmax | 0.85±0.34 | 0.47±0.09 | 0.49±0.09 | 1.83±0.63 | 1.73±0.30 |
| l SUVmean | 0.26±0.06 | 0.09±0.04 | 0.07±0.06 | 2.81±0.113 | 3.77±0.49 |
Table 1: The SUV values of different organs 30, 60 and 90 min post injection. Abbreviation: SUV = standardized uptake value.
| Group 1 (injury) | |||||
| Injury Achilles Tendon | Normal Achilles Tendon | Thigh muscle | Ratio(rupture/normal) | Ratio(rupture/muscle) | |
| 1 week | |||||
| l SUVmax | 0.38±0.17 | 0.22±0.11 | 0.11±0.06 | 1.77±0.65 | 2.05±0.78 |
| l SUVmean | 0.20±0.12 | 0.11±0.09 | 0.08±0.03 | 1.81±0.51 | 2.50±1.01 |
| 2 weeks | |||||
| l SUVmax | 0.59±0.38 | 0.23±0.15 | 0.17±0.13 | 2.56±0.81 | 3.47±1.12 |
| l SUVmean | 0.31±0.21 | 0.13±0.11 | 0.08±0.06 | 2.38±1.05 | 3.87±1.21 |
| 3 weeks | |||||
| l SUVmax | 0.43±0.15 | 0.26±0.11 | 0.19±0.17 | 1.65±1.01 | 2.26±0.73 |
| l SUVmean | 0.22±0.13 | 0.11±0.07 | 0.09±0.05 | 2.06±0.87 | 2.42±1.00 |
| Group2 (rupture) | |||||
| Rupture Achilles Tendon | Normal Achilles Tendon | Thigh muscle | Ratio(rupture/normal) | Ratio(rupture/muscle) | |
| 1 week | |||||
| l SUVmax | 0.41±0.17 | 0.21±0.18 | 0.33±0.17 | 1.95±0.91 | 1.25±0.71 |
| l SUVmean | 0.22±0.14 | 0.11±0.09 | 0.20±0.10 | 2.01±0.51 | 1.10±0.48 |
| 2 weeks | |||||
| l SUVmax | 0.63±0.27 | 0.29±0.07 | 0.18±0.04 | 2.19±1.21 | 3.47±0.87 |
| l SUVmean | 0.25±0.14 | 0.10±0.05 | 0.08±0.05 | 2.49±1.09 | 3.22±0.65 |
| 3 weeks | |||||
| l SUVmax | 0.55±0.41 | 0.28±0.08 | 0.26±0.10 | 1.99±0.61 | 2.08±0.86 |
| l SUVmean | 0.30±0.21 | 0.11±0.05 | 0.19±0.06 | 2.73±0.90 | 1.55±0.53 |
Table 2: The SUV value of different organs 1 week, 2 week, and 3 weeks post modeling. Abbreviation: SUV = standardized uptake value.
Discussion
The images show remarkable differences between the right and left sides. The small animal PET scans clearly illustrate the differences between the normal and injured/ruptured Achilles tendons. These differences can be attributed to the accumulation of FAPs on the injured/ruptured side leading to increased uptake of imaging agent. Imaging experiments along with measurements of SUVmax and SUVmean clearly demonstrate the contrast between the injured/ruptured Achilles tendon and the normal Achilles tendon. The alterations in FAP expression during the repair process can be effectively monitored using PET imaging.
The success of this protocol relies on several critical steps. First, the preparation and quality control of the [68Ga]-NOTA-FAP-2286 radiotracer are paramount. This involves employing precise radiochemical techniques to ensure high radiochemical purity and specific activity. Second, accurately inducing Achilles tendon injuries in rat models is crucial for maintaining consistency across subjects. Standardized surgical techniques and postoperative care should be according to established protocols. Third, determining the optimal timing for PET/CT imaging post injury is essential to effectively capture the most informative stages of the healing process. A series of time points may be necessary to accurately track the progression of FAP expression. Fourth, meticulous image acquisition and reconstruction protocols are required to maximize both signal-to-noise ratio and spatial resolution, which can be particularly challenging in small animal imaging studies9. Finally, rigorous quantitative analysis of PET/CT data plays a critical role in extracting meaningful information about the tendon healing process; this includes measurements such as SUV and potentially incorporating texture analysis. Additionally, the radiotracer exhibits rapid uptake and prolonged retention in tumors.
Several modifications and troubleshooting strategies can be considered to optimize the protocol. Adjustments in the radiotracer dose or imaging time points may be necessary to achieve an optimal signal-to-noise ratio, balancing sufficient uptake with minimal background signal. Refinement of the tendon injury model is essential to ensure consistency across subjects, potentially through standardizing the force applied in crush injuries or the extent of laceration. Implementation of motion correction techniques could be crucial in small animal imaging to minimize artifacts caused by respiratory or other involuntary movements. Exploring alternative image reconstruction algorithms, including iterative reconstruction methods, holds promise for enhancing spatial resolution and quantitative accuracy. Moreover, incorporating dynamic PET imaging could provide deeper insights into the kinetics of [68Ga]-NOTA-FAP-2286 uptake in injured tendons, thereby offering more comprehensive information about the healing process. NOTA-FAP-2286 also has demonstrated substantial potential in diagnosis and treatment of various diseases, particularly orthopedic conditions10. In malignant and benign bone tumors, such as osteosarcoma and metastatic bone tumors, NOTA-FAP-2286 offers high specificity for FAP, enabling precise PET/CT imaging and therapeutic applications11. Additionally, in inflammatory and fibrotic conditions such as joint fibrosis, fracture healing disorders, and arthritis, NOTA-FAP-2286 provides valuable insights into pathological processes and disease activity12.
Although innovative, this method has several limitations. The spatial resolution of PET imaging, typically ranging from 1 mm to 2 mm for small animal scanners, may restrict the ability to detect subtle changes in tendon healing, particularly during early stages or for small lesions10. There is a potential risk of non-specific radiotracer uptake in surrounding tissues, which could complicate result interpretation, especially in complex anatomical regions. Moreover, the requirement for specialized equipment and expertise in radiochemistry and small animal imaging may restrict its widespread adoption. Translating findings from rat models to human applications presents challenges due to variations in tendon size, healing processes, and injury scale. Additionally, although radiation exposure associated with PET imaging is minimal, it may limit the frequency of longitudinal studies on the same animal.
The significance of FAP-targeted PET/CT imaging lies in its potential to provide specific and sensitive molecular-level visualization of tendon healing processes9. Compared with conventional imaging techniques such as MRI or ultrasound, this technique offers unique insights into fibroblast activation, a crucial process in tendon healing. It allows for quantitative assessment of the progression of healing over time, potentially enabling earlier detection of abnormalities or complications in the healing process. Unlike other PET tracers like [18F]-FDG, which primarily reflects glucose metabolism, [68Ga]-NOTA-FAP-2286 specifically targets FAP, thereby providing more precise information on the fibroblastic response during tendon repair. This specificity enhances the ability to discern between normal healing and pathological processes, leading to more accurate lesion characterization.
The significance of this method in tendon injury research ismultifaceted. It provides a non-invasive approach to monitor the healing processes of tendons in preclinical studies, facilitating longitudinal assessment without the need for repeated biopsies or sacrificing animals at various time points. This not only significantly reduces the number of animals required for research but also yields more consistent data. Additionally, this method holds promise for evaluating novel therapeutic interventions for tendon injuries by offering a quantitative measure of treatment efficacy. Furthermore, it could be instrumental in investigating the molecular mechanisms underlying tendon repair and regeneration, potentially identifying new therapeutic targets. Particularly exciting is its potential translation to clinical applications, as it could enhance diagnosis and management of tendon injuries in humans. In the fields of orthopedic research, sports medicine, and regenerative medicine alike, this imaging technique has the potential to become a powerful tool for comprehending and optimizing strategies pertaining to tendon healing, ultimately leading to improved patient outcomes.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
The study was funded Zhejiang Medical and Health Science and Technology Program (grant No.2023KY694), Zhejiang Natural Science Foundation (grant number: LTG23H180014).
Materials
| Name | Company | Catalog Number | Comments |
| 68Ga Generator | Eckert&Ziegier | 10 84-470 | |
| C18 Cartridges | Waters | WAT020515 | |
| Chromatographic column | Waters | BEH C18 OBD Prep Column 5 μm,10 mm x 250 mm | |
| HPLC system | Agilent | DE63062140 | |
| Radioactivity detector | BIOSCAN,INC,WASHINGTON,D,C | B-FC-3200 PR 253212 | |
| Small animal PET | Siemens | SZ_200 |
References
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved