Method Article
Application of a Novel Hyaluronan Hydrogel for Three-Dimensional Follicle Culture and Methodology for Mouse Ovarian Follicle Cryopreservation
In This Article
Summary
This protocol describes a novel three-dimensional (3-D) culture model using a tyramine-linked hyaluronan hydrogel to encapsulate and culture preantral follicles from the mouse ovary. We also detail two approaches to ovarian follicle cryopreservation by vitrification.
Abstract
The 3-D architecture of the ovarian follicle and the complex interactions between somatic cell components and the oocyte that are necessary for cytoplasmic and nuclear maturation are difficult to maintain in conventional two-dimensional (2-D) culture systems. We describe a novel 3-D culture model using a tyramine-linked hyaluronan hydrogel for encapsulation and culture of mouse ovarian follicles. The hyaluronan encapsulation technique allows 3-D growth of follicles and retention of trophic factors in close proximity to the developing follicles. This hydrogel is highly versatile and can be applied to isolated follicles as well as ovarian tissue fragments. The viscoelastic properties of the HA gel enable adjustment of rigidity as well as moldability based on gel concentration. Preantral follicles developing in this culture model are able to complete meiotic maturation within 10-12 days of culture and ovulate a metaphase II oocyte upon triggering with hCG. This paper also details two approaches to ovarian follicle cryopreservation by vitrification.
Introduction
Human in vitro folliculogenesis remain a challenge even four decades after the first birth from in vitro fertilization. To date, methodology for human ovarian follicle culture that supports the production of a viable embryo resulting in a healthy baby is still lacking1. The optimal physical properties necessary for human in vitro follicle growth remain to be determined. The intact ovary is populated with thousands of follicles at various stages of development, and regulation of their growth is a complex process (Figure 1)2. Germinal vesicle stage (GV) oocytes from human preantral follicles take as long as 30 days in culture to become meiotically mature and reach the metaphase II stage3. Bidirectional communication between the oocyte and surrounding granulosa cells through gap junctions is critical for cytoplasmic and nuclear maturation4,5,6.
Conventional 2-D culture systems are not ideal for follicle culture, especially in larger mammalian models that require extended time in culture. Follicles attach to the dish, and the link between granulosa cells and the oocyte becomes more tenuous as granulosa cells migrate away. Three-dimensional (3-D) culture systems for follicles have therefore emerged as a means to more closely mimic in vivo physiology7,8.
Encapsulation of follicles within a matrix to promote 3-D growth has been one approach for preserving follicular architecture during in vitro culture (IVC). Biomatrices from natural polymers (such as collagen, agarose, fibrin, alginate, and hyaluronic acid), as well as synthetic polymers (such as polyethylene glycol, polyvinyl alcohol, and polyglycolic acid), have been tested7,9,10,11,12,13. The mechanical properties of a biomatrix have been shown to influence nutrient diffusion, thecal cell differentiation, antrum formation as well hormonal secretion14. Collagen, as a part of the cell's natural extracellular matrix (ECM), is one of the earliest matrices tested and was initially promising15,16,17. However, the logistics of standardizing preparations of collagen, poor mechanical properties, and stability have limited its use18. Agarose has been tested on cumulus-oocyte complexes (COCs) released from antral follicles as well as primordial follicles19,20. More recently, a printed 3-D agarose mold has shown promise for scaffold-free follicle culture21. Calcium alginate encapsulation, first reported in 2003, has, to date, been the most widely studied system for IVC22. It has been tested on mice, bovine, monkey as well as human follicles23,24,25,26,27. With calcium alginate, follicles are singly loaded into micro drops of the polymer and exposed to calcium chloride to generate a gel bead. Extraction of follicles from the bead requires treatment with a chelating agent. However, this matrix has some drawbacks. Alginate is a polysaccharide isolated from algae, and while it provides support, it is not part of the follicle's natural extracellular matrix. Data suggest a higher incidence of spindle defects after IVC in alginate28. Later modifications of the system by combining alginate with fibrin or other extracellular matrix components (ECM) have helped make the calcium alginate system more effective29,30.
Growing evidence points to the extracellular matrix as a key modulator in cell growth10,31,32 . It not only provides support but plays a critical role in cell attachment, function, growth, and communication. One of the major components of ECM is hyaluronan, a naturally occurring glycosaminoglycan. In the ovarian follicle, hyaluronan is produced by granulosa cells and contributes to the structural integrity and function of the developing follicle33,34. Integration of hyaluronan into a follicle culture model may therefore aid in creating a more physiological environment and enhance production of functionally competent oocytes.
This work describes the novel application of a tyramine-linked hyaluronan as a biomatrix for fresh and frozen ovarian follicle cultivation and in vitro maturation of oocytes (IVM). We also detail techniques for follicle cryopreservation by vitrification on two types of devices. One method involves direct immersion in liquid nitrogen whereas in the second method follicles are enclosed in a straw before immersion. The primary aim is to show that despite differences, both methodologies and devices can be reliably used for follicle cryopreservation.
Protocol
All animal experiments were carried out under Cleveland Clinic's institutional Animal Use and Care protocols and following the guidelines and regulations of the National Institutes of Health for Care and Use of Laboratory Animals.
1. Medium preparation
NOTE: The media described below will be used for the different steps in this procedure: ovarian tissue (OT) handling, OT collagenase digestion, follicle culture (FCM) and vitrification. Prepare all medium in a tissue culture hood using sterile technique.
- Ovarian tissue handling medium
- Supplement 20 mL of Leibovitz's medium (L-15) with 0.1% fetal bovine serum (FBS) in a 50 mL tissue culture flask (T-50).
- Tightly cap and place flask in incubator overnight to warm to 37 °C before use. This medium is used for handling ovarian tissue and follicles outside of the incubator and does not require CO2 to maintain pH of 7.2 to 7.4
CAUTION: Do not expose dishes to CO2 gas or the medium will become acidic.
- Collagenase digestion medium
- On morning of follicle harvest, add 1 mg of Type I collagenase (295 U/mg) to test tube with 2.2 mL of pre-warmed OT handling medium. Final desired concentration of collagenase is 134 U/mL Filter sterilize using 0.22 µm syringe filter. Tightly cap and place in warming block.
- Follicle culture medium and oil
- Prepare 30 mL of Minimum Essential Medium alpha supplemented with 5% fetal bovine serum in a T-50 flask. Supplement with 100 mIU/mL FSH, 10 mIU/mL LH, 10 µg/mL insulin, 5 µg/mL transferrin and 5 ng/m selenium.
- Pre-equilibrate FCM at 37 °C with 6% CO2 and air overnight in incubator before use.
- Place 50 mL of mineral oil in flask, loosely cap and equilibrate overnight in incubator.
- Vitrification media for FL and FL-Clusters
- The basal medium for all solutions is Global-Hepes supplemented with 20% synthetic protein substitute. Prepare 20 mL of VS1 solution with 7.5% ethylene glycol (EG) and 7.5% dimethyl sulfoxide (DMSO) in basal medium.
- Prepare 20 mL of VS2 with 15% EG, 15% DMSO and 0.5 M sucrose in basal medium. Sterilize all solutions with a 0.22 µm syringe filter and store at 4 °C until use. Solutions can be used for up to 4 weeks.
- Warming media for vitrified follicles and FL-Clusters
- The basal medium for all solutions is Global-Hepes supplemented with 20% synthetic protein substitute. Prepare 20 mL of basal medium with 0.25 M sucrose. Label as WS1.
- Prepare 20 mL of basal medium with 0.125 M sucrose and label as WS2. Filter sterilize with 0.22 µm syringe filter. Store solutions at 4 °C for up to 4 weeks.
2. Ovary harvest
- Euthanize 10-14-day old B6D2F1 pups by cervical dislocation (no anesthesia). Use 3-4 pups to obtain 250-300 intact preantral follicles for experimentation.
- Lay the animal on its back and swab belly with 70% isopropyl alcohol. Make a small horizonal cut at midline using clean scissors. Grasp skin above and below cut with fine forceps and pull in both direction (towards head and feet) to expose the abdomen.
- Using a second set of clean scissors and fine forceps, cut the abdominal wall. Lift the intestinal coils away. Locate the uterine horns, oviducts and ovaries. Excise ovaries and place in a center well dish containing 1 mL of OT handling medium warmed to 37 °C.
- Using a dissecting microscope, trim away any fat and oviductal tissue. Bisect the ovaries.
3. Follicle and FL- Cluster (FL-C) isolation
- Use a laminar flow hood with a 37 °C heated surface for follicle isolation and handling. Work aseptically in the hood. Be careful with the FCM dish. Use the bubbler to keep the dish gassed with 5% CO2 when working in the laminar flow hood.
NOTE: All solutions must be pre-warmed to 37 °C. FCM medium, as well as mineral oil, must be pre-equilibrated overnight in an incubator at 37 °C with 6% CO2 before use. Medium pH will shift when outside of the incubator for more than 10 min. - Pipet 6 mL of pre-equilibrated FCM into two 60 mm dishes and overlay with mineral oil. Place back in the incubator.
- Pipet 1 mL of collagenase in a center well dish and 3 mL of OT medium in the outer wall. Move ovaries to collagenase solution using a glass micropipette (1000 µm). Incubate the dish for 30-40 min on the heated surface of the laminar flow hood.
- At the end of the collagenase incubation, pipet 6 mL of OT medium into two 60 mm tissue culture dishes labeled 1 and 2. Place on the warm surface of the hood.
- Using a micropipette, move the collagenase-treated ovaries to the outer well to rinse free of collagenase. Change micropipettes and then move the ovaries to OT dish 1.
- Harvest follicles from each ovary using a P200 pipettor. Release follicles by repeated aspiration and expulsion of the enzyme-treated ovary through the pipet tip cut to different sizes. Mechanical teasing apart of tissue into fragments with two 27G needles before pipetting is also helpful in releasing individual follicles. Each mouse pup should yield 60-75 intact follicles of the desired size.
- If necessary, return undissociated pieces of ovary back into collagenase for another 5-10 min, rinse and repeat pipetting to release more follicles.
NOTE: Overexposure to collagenase will result in disruption of granulosa cell layers surrounding the oocyte. Do not try to break up the entire ovary. Stop once 250-300 follicles are collected. - Examine released follicles using a dissecting microscope at 40x magnification. Identify secondary pre-antral follicles (~120 -140 µm diameter) with a centrally located oocyte enclosed within an intact basement membrane (basal lamina). This size follicle will typically have 2-4 layers of granulosa cells surrounding the egg (See Figure 1).
NOTE: Compare follicles against opening of a 175 µm micropipette tip to approximate follicle size. The follicle should be sized to about 3/4 of the pipette diameter. This is the quickest method to select follicles of the desired size. - Using a 175 µm micropipette, move selected follicles to the OT2 dish. Upon completion of the collection, rinse all follicles in the FCM1 dish to remove any traces of the OT medium. Then transfer to the FCM2 dish.
- Place the dish in an incubator for 60 min before starting the embedding process.
- For follicle cluster isolation , perform steps 3.1 to 3.5. Use two tuberculin syringes (27 G) to tease apart the ovary into fragments and then into small clusters of 6-10 follicles.
NOTE: Culture of follicle clusters (FL-C) is an alternative to culturing individual follicles. This method retains native follicular architecture along with stromal components. Follicle size in FL-C is not uniform. Follicles retain their in vivo configuration with primordial, primary and secondary follicles being present. - Collect these follicle clusters (FL-C) with a 200 µm micropipette into the OT2 dish. Upon completion of collection, rinse all FL-C and transfer to FCM2 to await embedding.
4. Embedding follicles and follicle clusters
- Prepare a 10 mg/mL stock solution of activated tyramine-linked hyaluronan hydrogel (HA). Rehydrate 250 mg of tyramine substituted sodium hyaluronate powder with 25 mL of horseradish peroxidase enzyme (HRP; 10 IU/mL) in phosphate-buffered saline. Once solubilized, store 500 µL aliquots of this activated HA stock at -4 °C for future experiments.
- For follicle experiments, thaw the HA stock solution and dilute it to a concentration of 3 mg/mL in a global medium warmed to 37 °C. Perform all embedding steps in a laminar flow hood with a bench surface heated to 37 °C. Use a 60 mm dish with eight 100 µL wells for embedding and subsequent follicle culture.
- Move follicles or FL-clusters to be embedded from the FCM dish to a drop of HA gel to rinse free of culture medium. The tracking medium will interfere with gel formation. Place the FCM dish back under the bubbler to gas.
NOTE: Isolated follicles can be embedded, singly or in groups depending on the experiment. We generally prefer to seed 2-4 follicles per bead. - Place 1 µL of 0.03% hydrogen peroxide (H2O2) on a Petri dish. Add 25 µL of 3 mg/mL HA gel onto the hydrogen peroxide drop and mix by pipetting to initiate crosslinking (see Figure 2).
- Using a P20 pipettor, draw up the HA-H2O2 mix and pipet one drop (~8-10 µL) into two separate wells of the 8-well culture dish. Avoid making bubbles.
- Using a 200 µm micropipette, quickly transfer the follicles or an FL-C into the center of each drop (Figure 3). Speed is important as once the HA is exposed to the catalyst (peroxide) it will start to gel within 1-2 min. Be careful not to introduce bubbles during seeding.
NOTE: To track individual follicle growth, position the follicles at a distance from each other. Do not place follicles too close to the gel bottom or else during IVC they may descend enough to attach. - Allow ~ 3 min to complete the gelling process and then add 100 µL of pre-equilibrated FCM to each well. Repeat this process and load follicles into all eight wells. Overlay with warm pre-equilibrated mineral oil and place the dish in an incubator.
5. Vitrification of follicles and FL-clusters
NOTE: Vitrification can be done using either an open carrier (Cryoloop; CL), allowing direct contact with liquid nitrogen, or else a closed carrier (Rapid I; RI), where the sample is sealed within an outer straw and, therefore, never comes in contact with liquid nitrogen. Figure 4 shows the devices and contrasts of the two vitrification systems. Vitrification on both devices has been shown to be effective for embryo cryopreservation35.
- Closed carrier RI vitrification
- Pre-warm 2 mL aliquots of VS solutions to 37 °C in a heated block. Perform all vitrification steps on the heated surface of the laminar flow hood using a dissecting microscope to visualize the follicle and carrier.
- Fill the insulated cryo box with liquid nitrogen (LN2). Place the device's outer straw into a holding slot within the box so that it is partially immersed in liquid nitrogen. Lay the inner plastic stick with a tiny hole across the lid of a Petri dish in preparation for loading.
- Place two drops of VS1 side-by-side on a Petri dish near the top. Use a 200 µm micropipette to place two follicles in the first drop. Rinse and quickly move to the second drop of VS1. Incubate for 5 min. If vitrifying FL clusters, process a single cluster at a time.
- Place three drops of VS2 side-by-side on the same dish when the first incubation is almost complete. Then, quickly move follicles sequentially through the three VS2 drops within 60 s and load on the carrier. Be careful to draw a minimal amount of fluid with the follicles to avoid tracking the medium from one drop to another.
- To load the carrier, pick up two follicles and deposit with minimal fluid into the tiny hole in the plastic stick. The final fluid volume in the hole is minuscule < 0.5 µL. Avoid overfilling, which causes fluid to spill out of the hole and onto the plastic stick.
- Drop the stick into the pre-cooled outer straw. Use the ultrasonic sealer to close and seal the straw. Place the straw in a goblet attached to the cryocane. Up to 4 straws can be placed in the same goblet.
- Cover the cane with a plastic protective sleeve. Plunge the cane into a liquid nitrogen storage (LN2) tank.
- Open carrier CL vitrification
- Pre-warm VS solutions to 37 °C. Perform all vitrification steps on the heated surface of the laminar flow hood. Prepare the number of CL open carriers needed in advance. Insert the metal CL stem into the magnetized vial cap, making sure it is holding firmly (use a tiny dab of adhesive if necessary).
- Fill the insulated cryobox with liquid nitrogen (LN2). Place a rack into the cryobox to hold the cryovials such that LN2 is below the top of the vial.
- Fill the special magnetized and vented vial with LN2. Place it on the rack. Perform the vitrification steps as described in steps 6.1.3 to 6.1.5. The only difference is that we typically process five follicles at a time. Once again, be careful to minimize tracking fluid from drop to drop.
- To load follicles, grasp the CL open carrier by its attached magnetic cap using the metal wand. Dip the CL open carrier in a separate drop of VS2 to create a film of cryoprotectant.
- Using a micropipette, pick up all follicles or the FL-Cluster and place them on the film with minimal fluid. Work quickly, as follicles must be loaded before the film starts to dry.
- Immediately immerse the CL into the cryovial filled with LN2 to vitrify the sample. Cap and place vial on the cryocane. Cover with a plastic sleeve. Plunge the cane into a liquid nitrogen storage tank.
6. Warming of vitrified follicles and FL-clusters
- RI closed carrier warming
- Prepare a center well dish with 3 mL of pre-equilibrated FCM in the outer well and 1 mL in the center well. Overlay with oil and place in the incubator.
- Place 0.5 mL of pre-warmed WS1 and WS2 into two labelled centers well dishes. Move canes with samples from the storage tank into a cryobox filled with LN2.
- Remove the plastic cane cover. Remove the straw from the goblet, keeping it immersed, and slide it into the holding slot in the cryo box.
- Using fine scissors, cut the outer straw just above the black dot indicating the top of the inner RI carrier with follicles.
- Using fine forceps, lift the inner plastic stick slightly out of the outer straw. Grasp the device and quickly immerse the stick into WS1, gently swirling to unload follicles. Speed is critical. Follicles need to be unloaded into WS1 within 10 s.
- Use a dissecting microscope to visualize the follicles and to ensure that all have been unloaded from the carrier. After 2 min in WS1, use a micropipette to move all follicles (or FL-Cluster) to WS2, taking care not to track the medium.
- After 3 min, rinse follicles in the outer well of the FCM dish and then move to the center well. Place back in the incubator for 1-2 h before embedding.
- CL Open carrier warming
- Prepare FCM and WS dishes as described above. Move the cane with follicles from a storage tank into a cryo box filled with LN2. Take off the plastic cane cover.
- Use the magnetic wand to lift the cryovial cap until the metal stem of the CL is visible.
- Using forceps, grasp the metal stem. Remove the CL from the vial and very quickly immerse in WS 1, gently swirling to unload follicles. Unload into WS1 within 10 s. Using a dissecting scope, verify that all follicles have been unloaded
- All remaining steps for warming are the same as with RI closed carrier steps starting from step 7.1.6.
7. Follicle and FL-cluster imaging and media change
- Monitor follicles and FL-clusters in wells during the 10-12-day culture interval using an inverted light microscope with Hoffman contrast modulation optics and outfitted with a high-definition camera. Use imaging software to capture images at 40x and either 100x or 200x total magnification, depending on size.
- Imaging and assessment of follicles
- On culture Day 1, after embedding, image all culture wells at 40x and 200x magnification using an inverted microscope to establish baseline morphology and size. Return the dish to the incubator.
- View images and record the number of fully embedded follicles in each gel bead. For FL-C, make an estimate of follicle number at the start of culture.
- Using imaging software, measure follicle diameter along vertical and horizontal planes from the edge of the basement membrane. Measure oocyte size similarly from the outer edge of the zona. Record average values. Take a vertical and horizontal measure of cluster diameter.
- Continue to image cultures every 2-3 days. Measure the diameters of follicles not in contact with each other. With FL-C, the clusters ball up during IVC, so the entire cluster can be measured in the vertical and horizontal plane.
NOTE: For follicles grouped closely together, individual follicle borders may not be discernable after Day 4 of culture, so measurements are not taken. - Classify follicles becoming dark or apoptotic as well as those in which the oocyte is no longer surrounded by granulosa cells or has been extruded as non-viable. Keep track of any follicles or FL-C that have attached to the dish surface (see Figure 5).
- Carefully observe follicles for antrum formation from day 8 onwards. Follicles with antrums will look like they have a clearing or lighter space within them (Figure 6).
- Perform a half change of culture medium every 2 days. Use a P200 pipettor, set for 50 µL. Insert tip into well under the oil overlay and away from the gel bead. Slowly draw out 50 µL of the culture medium. Replace medium by slowly pipetting 50 µL of fresh FCM under the oil overlay. Avoid making bubbles.
8. Maturation of oocytes in encapsulated follicles
NOTE: The final maturation step is typically initiated when antrum formation amongst total seeded (and viable) follicles reaches over 40%. However, in the event that antrum formation is low or not visible, we recommend triggering by Day 12 of culture. We have observed no benefit in waiting any longer. For FL-C cultures, maturation is typically triggered when antrums are observed in 40% of wells or at the latest by Day 12.
- Prepare in vitro maturation medium (IVM) by supplementing FCM with 1.5 IU/mL of human chorionic gonadotrophin (hCG) and 5 ng/mL of epidermal growth factor (EGF).
- Trigger maturation by replacing FCM in each well with 100 µL of IVM medium at around 5 PM. After overnight maturation (16-18 h after trigger), use a dissecting microscope at 40x magnification to examine each culture well for cumulus-oocyte complexes (COCs) that have ovulated from the HA gel bead. The COCs are usually found resting just above the gel bead or else in close proximity.
- Collect the ovulated COCs into a center well dish with 1 mL of pre-equilibrated FCM overlayed with oil and place the dish back in the incubator.
- Collect oocytes from follicles that are still embedded by gently pipetting the HA-bead using a P200 pipettor to release non-ovulated COCs. Collect these in a separate dish with FCM.
- Prepare a center well dish with 1 mL of hyaluronidase (10 IU/mL) and 3 mL of FCM medium in the outer wall.
- Transfer ovulated COCs into hyaluronidase solution and briefly expose to the enzyme (30-45 s) to denude the oocyte of granulosa cells in order to visualize oocyte nuclear status. Rinse oocytes to remove enzyme before placing in 5 µL drops of fresh medium under oil for detailed assessment. Place the dish in incubator. Repeat this process for the non-ovulated COCs.
- Record the total number of ovulated and non-ovulated eggs recovered. Photograph and assess nuclear status (GV, metaphase I or metaphase II) of each recovered oocyte. Measure and record diameter.
- Calculate IVC survival rate based on total viable follicles (those making it to the day of hCG trigger) and total eggs recovered. Calculate the percentage of metaphase II oocytes from the ovulated COCs. Repeat this for any oocytes recovered from the non-ovulated COCs.
- Determine maturation rate to metaphase II in same manner for FL-C.
Results
This paper details methodology for using a novel tyramine linked hyaluronan gel for in vitro culture of mouse preantral follicles36,37. Figure 6 illustrates the differences between preantral follicle growth when placed in a conventional 2-D culture system versus a single follicle encapsulated in HA gel for 3-D culture. The native follicle architecture is maintained during the 12 days of culture with an antrum clearly visible on the last day of growth.
The HA gel is very versatile, allowing the growth of isolated follicles singly or in groups and also ovarian tissue is mechanically broken into small clusters of follicles. The gel is transparent making it possible to visualize follicles even if at different depths. Encapsulated follicles and FL-C exhibit radial expansion from continued granulosa cell proliferation (Figure 7). Initial follicle diameters average 139.8 ± 28 µm with the GV oocyte diameter measuring 63.5 ± 4.6 µm. In singly cultured follicles, final diameters measure at about 385.6 ± 36.7 µm, a roughly 3-fold increase in size. Ovulated metaphase II oocytes measure around 84.8 ± 3.8 µm. Within cultured FL-clusters, follicle size is fairly diverse (Figure 5, Figure 7). Ovulated oocytes after hCG trigger are found near the follicle (Figure 8). The majority of metaphase II oocytes will be retrieved from the ovulated COCs. Follicles still embedded after trigger usually contain GV and metaphase I oocytes.
Table 1 contrasts maturation rates between isolated follicles and FL-clusters from fresh or frozen ovaries. FL-C from cryopreserved ovaries had significantly lower maturation rates. Microscopic observations showed them to frequently have broken basal lamina, making them quite susceptible to premature oocyte extrusion. The fragile nature of follicle clusters was somewhat countered by encapsulation. Collagenase treatment of cryopreserved ovaries was avoided as it was especially damaging with low survival and low yield of intact follicles.
Cryopreservation of isolated follicles is much more effective than whole ovary preservation. High maturation rates can be achieved with both vitrification methods examined (Table 2). Despite large differences in cooling rates, oocyte maturation after IVC did not differ. The CL open carrier does allow for more efficiency as up to ten follicles can be loaded on a single CL open carrier. This also shortens the overall time for recovering multiple cryopreserved follicles. However, for any eventual clinical application of vitrification for human follicles the closed sealed system may be preferable.
Chromatin arrangement around the nucleolus of the GV oocyte can be used to identify oocytes most likely to fertilize after ovulation and develop to blastocysts38. Figure 9 illustrates live staining of oocytes to visualize the chromatin distribution pattern.

Figure 1: Schematic of follicle growth. This diagram illustrates the different stages of follicle development from a primary follicle to the secondary preantral stage and finally to a fully mature tertiary follicle ready for ovulation. A microscopic image of a typical preantral follicle is also shown with its different morphologic features. Please click here to view a larger version of this figure.

Figure 2: Schematic of HA encapsulation method. Structure of the hyaluronan gel and the different steps for follicle embedding are illustrated in this diagram. Please click here to view a larger version of this figure.
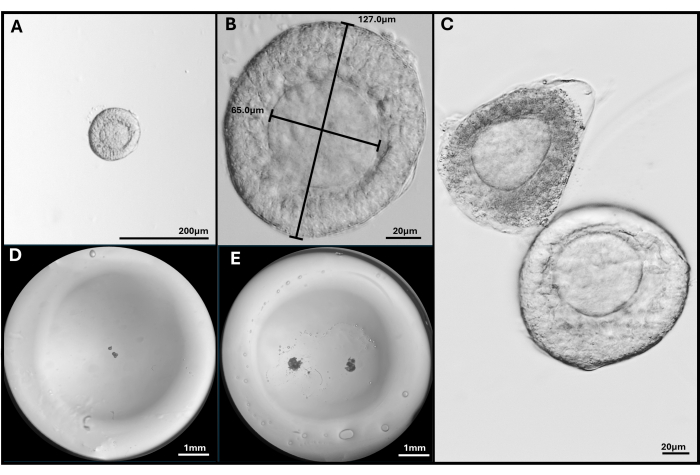
Figure 3: Follicle isolation and encapsulation. (A, B) Preantral follicle selected for embedding at magnification 40x and 200x. (C) Apoptotic follicle is shown with healthy preantral follicle with oocyte not quite central. (D) Image of HA gel bead seeded with follicles and (E) with two FL-C. Images taken with a stereomicroscope to show the entire gel bead. Please click here to view a larger version of this figure.
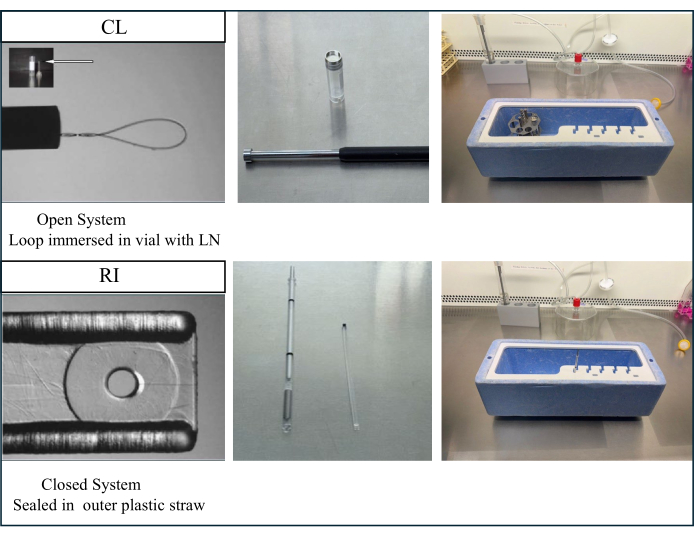
Figure 4: Vitrification devices for cryopreservation of isolated follicles. With the CL open carrier device, the vitrification step is conducted by direct immersion of follicles into liquid nitrogen. The rate of cooling is, therefore, extremely high, over -20,000 °C/min. In contrast, with the RI closed carrier, follicles are loaded on the inner plastic stick and dropped into an outer straw immersed in liquid nitrogen. This closed vitrification method avoids direct contact with liquid nitrogen. However, the cooling rate is significantly lower at -1220 °C/min. Loading and recovery of follicles from either carrier is easy. The CL open carrier accommodated loading up to ten follicles per device as compared to just two with the RI closed carrier. This figure has been modified from35. Please click here to view a larger version of this figure.

Figure 5: Representative images of problems encountered. (A) Follicle cluster with oocyte being extruded. (B) Isolated follicles with broken basal lamina membrane and one with an extruded oocyte. (C) Embedded follicle under a bubble in the gel. (D) Follicle cluster that remained in gel (left) compared to cluster embedded too deep that eventually attached to the dish. The wide range of follicle sizes in FL-C is clearly visible. Please click here to view a larger version of this figure.

Figure 6: Comparison of follicle growth in conventions 2-D versus 3-D culture in HA. With 2-D growth, flattening of the follicle and attachment of granulosa cells to the tissue culture dish was observed by Day 4, leaving the oocyte vulnerable to granulosa cell migration, disruption of gap junctions, and premature oocyte extrusion. The HA-encapsulated follicle remained unattached throughout the culture interval. Granulosa cell expansion occurred in all directions, encasing the oocyte and maintaining 3-D architecture. This figure has been modified from36. Please click here to view a larger version of this figure.

Figure 7: Representative images of follicles encapsulated in tyramine-linked hyaluronan gel. (A) Preantral follicle collected after collagenase digestion of fresh ovary on Day 1. (B) Gel drop seeded with four preantral follicles imaged on Day 1 and (C) Day 4 of culture (D) Follicle cluster from fresh ovary on day 2 (E) on Day 6 and (F) on Day 9 of culture. (G) Follicle cluster mechanically dissected from vitrified whole ovary shown on Day 2 and (H) on Day 6 of culture. (I) Follicle with antrum formation is clearly visible on Day 9 of culture. Please click here to view a larger version of this figure.
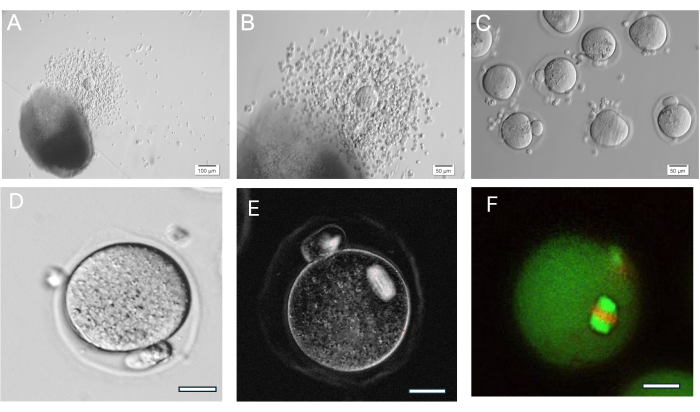
Figure 8: Oocyte ovulation. (A, B) Ovulated cumulus-oocyte complex (COC) shown next HA-gel bead. (C) Oocytes were imaged after enzymatic treatment of COCs with hyaluronidase to remove surrounding cumulus cells. Numerous metaphase II oocytes. (D) Metaphase II oocyte with prominent polar body. Magnification 400x. (E) Live imaging of metaphase II oocyte using polarized light and an imaging system to visualize meiotic spindle and assess organization, done as described in37. Magnification 400x. Normal birefringent spindle visible. (F) Metaphase II oocyte fixed and stained with anti-alpha/beta-tubulin and propidium iodide to visualize meiotic spindle organization. Please click here to view a larger version of this figure.

Figure 9: Chromatin reorganization in GV oocytes. Chromatin arrangement in GV oocytes after antrum formation was examined by staining of DNA with Hoechst 33342 (50 ng/mL). Representative follicles were harvested from HA beads by gentle pipetting. Granulosa cells were removed using hyaluronidase. GV oocytes were then stained for 15 min. (See protocol by Monti et al.38). Images were taken at 40x magnification. (A) GV oocyte shown on Day 1 at culture initiation exhibiting the non-surrounded chromatin (NSN) staining pattern. (B) GV oocyte from growing follicle with antrum shown on the day of hCG trigger. Chromatin condensed and formed a perinuclear ring around the nucleolus. Please click here to view a larger version of this figure.
| Parameter | Fresh Ovary | Frozen Ovary | |
| FL-Isolated | FL-Cluster | FL-Cluster | |
| Follicles Observed During IVC | 130 | 154 | 69 |
| Ovulation after HCG (%) | 71% | 66% | 93% |
| (92/130) | (101/154) | (64/69) | |
| GVBD (%) | 30% | 28% | 52% |
| (28/92) | (28/101) | (33/64) | |
| MII oocyte formation (%) | 59% | 55% | *34% |
| (54//92) | (56/101) | (22/64) | |
Table 1: Outcomes with HA-embedded follicles from fresh and vitrified ovaries. Follicles and FL-C from fresh ovaries were matured in vitro after encapsulation in HA gel. HA gel was also tested on follicles from ovaries vitrified using an EG/DMSO protocol39. With fresh ovaries, both individual follicles (FL) and follicle clusters (FL-C) were collected after collagenase digestion. For vitrified ovaries, exposure to collagenase was, in fact, damaging to follicles. The best approach with cryopreserved ovaries was to isolate FL-clusters rather than individual follicles and to only use mechanical dissection with needles. The table contrasts outcomes between HA beads seeded with follicles in groups of 4-6 versus beads with a single FL-C containing 6-10 follicles. *The maturation rate with FL-C from cryopreserved ovaries was significantly lower (p = 0.008; Chi-Square analysis to test for significance).
| Carrier | RI | CL |
| (Closed) | (Open) | |
| Survival (%) | 100% (24/24) | 100% (41/41) |
| Antrum formation* (%) | 25.0% (6/24) | 75.6% (31/41)* |
| Ovulation rate (%) | 66.7% (16/24) | 87.8% (36/41) |
| Maturation rate (% MIIs) | 81.3% (13/16) | 69.4% (25/36) |
Table 2: Outcomes after cryopreservation of isolated follicles on two different vitrification devices. High maturation rates were achieved with both the open CL device as well as the closed RI carrier, with its lower cooling rate. Antrum formation was the only outcome measure observed to be significant but did not impact the overall maturation rate (p < 0.05; Chi-square analysis to test for significance).
Discussion
The ability to control the mechanical properties and biodegradability of the tyramine-linked HA hydrogel offers many advantages for tissue engineering applications. Our laboratory is the first to apply this specific HA gel for ovarian follicle growth. This patented tyramine-substituted sodium hyaluronate gel (TS-NAHY) is a novel hyaluronan-based hydrogel system developed at the Cleveland Clinic. Crosslinking of the gel is driven by exposure of the peroxidase in the activated gel mix to an oxidizing agent. This can be done both in vitro or in vivo. Formed TS-NAHY hydrogels display a wide spectrum of properties from weak gel, a paste to a fragile solid, depending on the concentration of gel40.
The disappointing progress with ovarian follicle culture highlights the need to design new culture models. Creation of a culture system based exclusively on native extracellular matrix components may be a more advantageous approach. The HA culture model described is easy to use in a physiological manner with no requirement for additional ECM components. The gel is transparent, allowing detailed visualization of follicles. The viscoelastic properties of the HA gel facilitate the adjustment of rigidity as well as moldability. This feature increases the versatility of this biomatrix. Rigidity of a biomatrix can impact granulosa cell proliferation and antrum formation30,41,42. Follicles derived from culture environments permissive to antrum formation have been reported to have different gene expression profiles than those in a non-supportive culture system43. Primate follicles appear to require a more rigid matrix44. Tailoring the biomatrix to meet the requirements of different animal species, including humans, will likely be important for successful in vitro maturation.
For mouse preantral follicle growth, we have tested gel concentrations ranging from 2-5 mg/mL36. Oocyte maturation rates ranged from 44% to 58%. Higher concentrations of the HA gel allowed more moldability and retention of a 3-D structure, but follicle expansion was affected. Lower HA concentrations permitted more radial expansion of the follicle but increased the risk of the follicle being spontaneously extruded before the end of IVC. The 3-3.5 mg/mL concentration of HA worked best for mouse preantral follicles. Keeping the gel drop small was essential for forming HA gel beads with sufficient depth to keep follicles in a 3-D configuration throughout IVC. Larger drops flattened, resulting in the descent of follicles through the gel and attachment to the plate surface. One limitation of the use of this biomaterial for embedding is the extremely fast gelation time. It makes seeding more than two HA gel beads at a time difficult. Increasing the efficiency of follicle seeding into the HA gel is one area we are trying to improve on.
The culture of follicle clusters in a 3-D environment using HA has great potential. Normal ovarian architecture is retained, with different-sized follicles in contact with each other and the supporting stroma. LH may help the growth of smaller preantral follicles in the FL-C by inducing changes in the early differentiating thecal cells, so it was included in the FCM medium45. Continuing the culture of FL-C past 12 days to see if a new wave of growth can be initiated in any of the smaller follicles still embedded after the hCG trigger needs further study. Further optimization of the culture milieu may be a prerequisite for the cultivation of follicles of different size ranges in a tissue fragment. An advantage of this 3-D HA culture model with FL-C is that it allows close mimicking of in vivo follicular arrangement and interactions. Another important attribute is that unlike calcium alginate and other polymer systems, ovulation and maturation can be induced without physically removing the follicles from either the tissue or the gel matrix.
The time needed for in vitro follicle culture and obtaining mature oocytes for freezing is long, especially in large mammals. The ability to cryopreserve harvested follicles or follicle clusters presents a way to delay this step until a later and possibly more favorable time. If such technology can someday be applied to human follicles during the ovarian harvest, it may be beneficial. Whole ovary cryopreservation for fertility preservation is, at present, the only option for patients. But whether it is, in fact, the best method remains to be determined. In this paper, we present a vitrification methodology for preantral follicle cryopreservation that gives excellent post-warming survival and maturation rates after 3-D culture.
In conclusion, we have described a new 3-D culture model using hyaluronan, a component of native ECM. The HA encapsulation technique allows the retention of trophic factors in close proximity to the developing follicles. The methodology for encapsulation in the biomatrix is simple and can accommodate isolated follicles as well as follicle clusters. The latter may open new avenues of research and provide insight into the fundamental biology of folliculogenesis and its regulation. Oocytes from HA-encapsulated follicles have been shown to be functionally competent37. These oocytes can be fertilized, form blastocysts in vitro, and implant upon transfer to pseudo-pregnant mice. These data validate the use of tyramine-linked hyaluronan as a biomatrix for 3-D follicle culture and in vitro oocyte maturation. This protocol could potentially be applied to ovarian follicle culture in other animal models, including humans. Other possible applications for this 3-D HA gel system might be embryoid body and organoid culture.
Disclosures
No conflicts of interest or disclosures.
Acknowledgements
We want to acknowledge the entire embryology team at Cleveland Clinic for their assistance as well as the REI department and especially Dr. Falcone for support. This project was funded through a research fund at the Cleveland Clinic.
Materials
| Name | Company | Catalog Number | Comments |
| Anti-alpha tubulin-FITC labelled | Sigma-Aldrich | F2168 | |
| Anti-beta tubulin-FITC labelled | Sigma-Aldrich | F2043 | |
| BZ-X700 | Keyence | ||
| Center well dish | Fisher Scientific | 08-772-12 | |
| Collagenase Type I | Worthington Biochemical Corporation | LS004196 | |
| Crycap vial-vented | Hampton Research | HR4-904 | |
| Cryoloop | Hampton Research | HR4-974 | |
| Crystal cap | Hampton Research | HR4-733 | |
| Culture dish 60mm | Fisher Scientific | 08-772B | |
| Dimethyl sulfoxide (DMSO) | Sigma-Aldrich | D2650 | |
| Epidermal growth factor (EGF) | R &D Systems | 236-EG | |
| Ethylene Glycol (EG) | Sigma-Aldrich | 293237 | |
| Fetal Bovine Serum-Heat Inactivated | ThermoFisher Scientific | 10082-147 | |
| Follicle Stimulating Hormone (FSH) | Sigma-Aldrich | F4021 | |
| Global-Hepes medium | CooperSurgical | LGGH-100 | |
| Hoechst 33342 | Sigma-Aldrich | B2261 | |
| Human chorionic gonadotrophin (hCG) | Sigma-Aldrich | CG10 | |
| Human serum albumin | CooperSurgical | GHSA-125 | |
| Hyaluronidase | CooperSurgical | ART-4007-A | |
| Hydrogen Peroxide | CVS Pharmacy Inc. | 372441 | |
| Insulin-transferrin-selenium (ITS) | ThermoFisher Scientific | 41400-045 | |
| Leibovitz medium (L-15) | ThermoFisher Scientific | 11415-064 | |
| Luteinizing hormone | Sigma-Aldrich | L9773 | |
| Magnetic wand | Hampton Research | HR4-729 | |
| Micropipettes (1000 µm) | Minitube | 19025/0050 | |
| Micropipettes (175 , 200, and 275µm) | CooperSurgical | MXL3-175, MXL3-200, MXL3-275 | |
| Millex GV filter 0.22 µm | Millipore | SLGU033RS | |
| Mineral oil | CooperSurgical | LGOL-500 | |
| Minimum Essential Medium alpha (MEM) | ThermoFisher Scientific | 32561-037 | |
| Oocyte Imaging System-Spindleview | Hamilton Thorne | ||
| Phosphate buffered saline (PBS) | ThermoFisher Scientific | 10010-023 | |
| Propidium iodide | Sigma-Aldrich | P4170 | |
| Rapid i | VitroLife | 14406 | |
| SmartBox | VitroLife | 14423 | |
| Synthetic Protein Substitute (SPS) | CooperSurgical | ART-3011 | |
| Tyramine -linked Hyaluronan Biohydrogel Kit | LifeCore | ENG-00151 | |
| Ultrasonic sealer | VitroLife | 14415 | |
| Universal GPS Culture dish 8x 100 µl wells | CooperSurgical | UGPS-010 |
References
- Telfer, E. E., Andersen, C. Y. In vitro growth and maturation of primordial follicles and immature oocytes. Fertil Steril. 115 (5), 1116-1125 (2021).
- Telfer, E. E., McLaughlin, M. Natural history of the mammalian oocyte. Reprod Biomed Online. 15 (3), 288-295 (2007).
- Xiao, S., et al. In vitro follicle growth supports human oocyte meiotic maturation. Sci Rep. 5, 17323 (2015).
- Carabatsos, M. J., Sellitto, C., Goodenough, D. A., Albertini, D. F. Oocyte-granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev Biol. 226 (2), 167-179 (2000).
- Diaz, F. J., Wigglesworth, K., Eppig, J. J. Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice. Dev Biol. 305 (1), 300-311 (2007).
- Eppig, J. J., Pendola, F. L., Wigglesworth, K., Pendola, J. K. Mouse oocytes regulate metabolic cooperativity between granulosa cells and oocytes: amino acid transport. Biol Reprod. 73 (2), 351-357 (2005).
- Dadashzadeh, A., Moghassemi, S., Shavandi, A., Amorim, C. A. A review on biomaterials for ovarian tissue engineering. Acta Biomater. 135, 48-63 (2021).
- Simon, L. E., Kumar, T. R., Duncan, F. E. In vitro ovarian follicle growth: a comprehensive analysis of key protocol variablesdagger. Biol Reprod. 103 (3), 455-470 (2020).
- Belli, M., et al. Towards a 3D culture of mouse ovarian follicles. Int J Dev Biol. 56 (10-12), 931-937 (2012).
- Berkholtz, C. B., Shea, L. D., Woodruff, T. K. Extracellular matrix functions in follicle maturation. Semin Reprod Med. 24 (4), 262-269 (2006).
- Desai, N., et al. Three-dimensional in vitro follicle growth: overview of culture models, biomaterials, design parameters and future directions. Reprod Biol Endocrinol. 8, 119 (2010).
- Shea, L. D., Woodruff, T. K., Shikanov, A. Bioengineering the ovarian follicle microenvironment. Annu Rev Biomed Eng. 16, 29-52 (2014).
- Paulini, F., et al. Survival and growth of human preantral follicles after cryopreservation of ovarian tissue, follicle isolation and short-term xenografting. Reprod Biomed Online. 33 (3), 425-432 (2016).
- West, E. R., Xu, M., Woodruff, T. K., Shea, L. D. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 28 (30), 4439-4448 (2007).
- Joo, S., et al. The effect of collagen hydrogel on 3D culture of ovarian follicles. Biomed Mater. 11 (6), 065009 (2016).
- Telfer, E., Torrance, C., Gosden, R. G. Morphological study of cultured preantral ovarian follicles of mice after transplantation under the kidney capsule. J Reprod Fertil. 89 (2), 565-571 (1990).
- Torrance, C., Telfer, E., Gosden, R. G. Quantitative study of the development of isolated mouse pre-antral follicles in collagen gel culture. J Reprod Fertil. 87 (1), 367-374 (1989).
- Dong, C., Yonggang, L. V. Application of collagen scaffold in tissue engineering: recent advances and new perspectives. Polymers. 8 (2), 42 (2016).
- Le, B. A. M., et al. Agarose-based 3D culture improved the developmental competence of oocyte-granulosa complex isolated from porcine preantral follicle. Theriogenology. 223, 11-21 (2024).
- Park, J. E., et al. In vitro maturation on an agarose matrix improves the developmental competence of porcine oocytes. Theriogenology. 157, 7-17 (2020).
- Zaniker, E. J., et al. Three-dimensionally printed agarose Micromold supports scaffold-free mouse ex vivo follicle growth, ovulation, and luteinization. Bioengineering. 11 (7), 719 (2024).
- Pangas, S. A., Saudye, H., Shea, L. D., Woodruff, T. K. Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Eng. 9 (5), 1013-1021 (2003).
- West, E. R., Shea, L. D., Woodruff, T. K. Engineering the follicle microenvironment. Semin Reprod Med. 25 (4), 287-299 (2007).
- Xu, J., et al. Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three-dimensional culture: effects of gonadotropins and insulin. Reproduction. 140 (5), 685-697 (2010).
- Xu, M., Kreeger, P. K., Shea, L. D., Woodruff, T. K. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 12 (10), 2739-2746 (2006).
- Amorim, C. A., Van Langendonckt, A., David, A., Dolmans, M. M., Donnez, J. Survival of human pre-antral follicles after cryopreservation of ovarian tissue, follicular isolation and in vitro culture in a calcium alginate matrix. Hum Reprod. 24 (1), 92-99 (2009).
- Converse, A., Zaniker, E. J., Amargant, F., Duncan, F. E. Recapitulating folliculogenesis and oogenesis outside the body: encapsulated in vitro follicle growth dagger. Biol Reprod. 108 (1), 5-22 (2023).
- Mainigi, M. A., Ord, T., Schultz, R. M. Meiotic and developmental competence in mice are compromised following follicle development in vitro using an alginate-based culture system. Biol Reprod. 85 (2), 269-276 (2011).
- Kreeger, P. K., Deck, J. W., Woodruff, T. K., Shea, L. D. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 27 (5), 714-723 (2006).
- Shikanov, A., Xu, M., Woodruff, T. K., Shea, L. D. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials. 30 (29), 5476-5485 (2009).
- Griffith, L. G., Swartz, M. A. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 7 (3), 211-224 (2006).
- Irving-Rodgers, H. F., Rodgers, R. J. Extracellular matrix of the developing ovarian follicle. Semin Reprod Med. 24 (4), 195-203 (2006).
- Salustri, A., Camaioni, A., Di Giacomo, M., Fulop, C., Hascall, V. Hyaluronan and proteoglycans in ovarian follicles. Hum Reprod Update. 5, 293 (1999).
- Rashki Ghaleno, L., Cristian, P. P., Shahverdi, A., Dardmeh, F., Alipour, H., Valojerd, M. R. Exploring the role of hyaluronic acid in reproductive biology and beyond: Applications in assisted reproduction and tissue engineering. Adv. Biology. 8 (6), e202300621 (2024).
- Desai, N. N., Goldberg, J. M., Austin, C., Falcone, T. The new Rapid-i carrier is an effective system for human embryo vitrification at both the blastocyst and cleavage stage. Reprod Biol Endocrinol. 11, 41 (2013).
- Desai, N., Abdelhafez, F., Calabro, A., Falcone, T. Three dimensional culture of fresh and vitrified mouse pre-antral follicles in a hyaluronan-based hydrogel: a preliminary investigation of a novel biomaterial for in vitro follicle maturation. Reprod Biol Endocrinol. 10 (1), 29 (2012).
- Desai, N., Spangler, M., Nanavaty, V., Gishto, A., Brown, A. New hyaluronan-based biomatrix for 3-D follicle culture yields functionally competent oocytes. Reprod Biol Endocrinol. 20 (1), 148 (2022).
- Monti, M., Redi, C. A. Isolation and characterization of mouse antral oocytes based on nucleolar chromatin organization. J Vis Exp. (107), e53616 (2016).
- Huang, L., et al. Cryopreservation of human ovarian tissue by solid-surface vitrification. Eur J Obstet Gynecol Reprod Biol. 139 (2), 193-198 (2008).
- Chan, J., Darr, A., Alam, D., Calabro, A. Investigation of a novel cross-linked hyaluronan hydrogel for use as a soft-tissue filler. Am J Cosmetic Sur. 22, 105-108 (2005).
- Shikanov, A., Xu, M., Woodruff, T. K., Shea, L. D. A method for ovarian follicle encapsulation and culture in a proteolytically degradable 3-dimensional system. J Vis Exp. (49), e2695 (2011).
- Xu, M., West, E., Shea, L. D., Woodruff, T. K. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 75 (6), 916-923 (2006).
- West-Farrell, E. R., et al. The mouse follicle microenvironment regulates antrum formation and steroid production: alterations in gene expression profiles. Biol Reprod. 80 (3), 432-439 (2009).
- Xu, M., et al. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 81 (3), 587-594 (2009).
- Wu, J., Nayudu, P. L., Kiesel, P. S., Michelmann, H. W. Luteinizing hormone has a stage-limited effect on preantral follicle development in vitro. Biol Reprod. 63 (1), 320-327 (2000).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved