Method Article
Dynamic Multiparameter Platelet Function Assessment Using a Capacitive Biosensor
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
A protocol for a novel dynamic multiparameter platelet functional assay using a capacitive biosensor is presented here. This approach, designed within a semi-rigid microenvironment to enhance physiological relevance, provides three output parameters sensitive to platelet count, stimulation strengths, and activation pathways.
Streszczenie
Platelets play a fundamental role in blood clotting through a series of regulated responses, including adhesion, spreading, granular secretion, aggregation, and cytoskeletal contraction. However, current assays are limited to partial analysis of platelet function under non-physiological conditions. Thus, an improved assay that reflects the dynamic and multifaceted nature of platelet function in physiological settings is necessary. In this context, a novel approach is introduced to measure several key parameters related to platelet function in a more physiologically relevant ex vivo semi-rigid microenvironment compared to traditional assays. This method utilizes an advanced electrical biosensor, the membrane capacitance sensor (MCS), which provides unique insights into the clotting process through three distinct readouts. These readouts are highly sensitive to variations in platelet count, stimulation intensity, and specific activation pathways. As a purely electrical sensing platform, the MCS demonstrates significant potential as a diagnostic tool for detecting primary hemostatic function disorders, evaluating the efficacy of therapeutic treatments, and advancing the broader understanding of the roles of platelets in hemostasis and thrombosis.
Wprowadzenie
Platelets, specialized blood cells, are pivotal in orchestrating the hemostatic response to halt bleeding following injury and in facilitating the healing of blood vessels1. Additionally, they also serve as crucial mediators in thrombosis, a leading cause of thromboembolic disease-related deaths globally2,3,4,5,6. When a vascular injury occurs, platelets undergo a series of complex, regulated, and multi-stage functional processes. These include adhesion to the intimal matrix, an influx of intracellular calcium triggering platelet conformational changes, activation, granule secretion, aggregation, and cytoskeletal contraction, ultimately forming and stabilizing hemostatic plugs to seal the damaged sites and prevent bleeding7,8. Despite significant advancements in antiplatelet drugs and therapeutic strategies9,10,11, thrombosis risk persists. Antiplatelet therapy management presents challenges, including the risk of iatrogenic bleeding, difficulty in achieving antithrombotic efficacy while maintaining hemostasis, and variability in patient responsiveness, including drug resistance12,13.
Although the molecular mechanisms governing platelet response phases are well documented, current methods for testing platelet function remain suboptimal. Traditional laboratory-based tests often fall short as they only assess limited aspects of early-to-mid-stage platelet activity, such as adhesion, aggregation, or clot viscosity7,14,15,16,17,18. This partial analysis can lead to insufficient information. Moreover, these tests do not offer simultaneous, continuous, and rapid assessment of multiple crucial platelet functional elements within a single assay. Consequently, this limitation hampers advancements in both clinical and experimental hematology. Over time, a plethora of impedimetric or capacitive sensors have been developed for various biomedical applications19,20,21,22,23,24,25,26,27,28.
Here, we present the protocol for a multiplexed platelet function assessment using a capacitive biosensor. The proposed approach offers an attractive feature by sensitively monitoring dynamic changes in a broad spectrum of platelet functions at the cellular level. The presented approach utilizes a biosensor comprised of two microchips: a top-silicone chip, which is disposable, featuring a sample well for citrated platelet-rich plasma and a sensing electrode coated with human Fibronectin to facilitate platelet adhesion, alongside a reusable bottom silicon chip housing a reference electrode. Continuous measurement of dynamic capacitance changes during the whole coagulation process, encompassing platelet adhesion, activation, and post-activation, enables sensitive analysis linked to variations in platelet counts, levels of platelet activation, and the inhibition of activation pathways. The clinical feasibility and utility of this method were shown using pertinent human plasma samples, underscoring its potential for robust platelet function assessment in clinical settings.
Protokół
The study proposal was approved by the Human Subjects Division (HSD) at the University of Washington Internal Review Board (UW-IRB; Study ID: STUDY00005211). All volunteer subjects who participated in the study provided written informed consent. The details of the reagents and equipment used in this study are listed in the Table of Materials.
1. Fabrication steps for the membrane capacitive sensor (MCS)
NOTE: The MCS sensor was fabricated utilizing traditional microfabrication techniques. This biosensor was composed of a top (T-) and bottom (B-) membrane capacitance chip (MCC). Briefly, the fabrication steps are shown in Figure 1.
- Microfabrication steps for T-MCC (Figure 1A)
- Design the layout of the T-MCC using standard CAD layout software for a 4-inch silicon wafer substrate.
- Transfer the design onto a chromium glass photomask using the standard mask fabrication process29,30.
- On a clean double-side polished silicon substrate (400µm thick), deposit 500-nm-thick of Si3N4 using standard low-pressure chemical vapor deposition (LPCVD) technique31. Spin coat 12 µm of AZ 9260 photoresist at 2000 rpm for 25 s using a manual spin coater.
- Using standard UV photolithography technique32, pattern the photoresist, which involves exposing the photoresist with the chrome glass photomask in a UV aligner, and developing the resist in a developer solution to remove the UV-exposed region of the resist.
- Pattern the underlying 500-nm-thick silicon nitride layer using a reactive ion etching process, which exposes the silicon substrate.
- Remove the exposed 400µm thick silicon substrate using a standard Bosch process: Deep Reactive Ion Si Etch process33.
- Calculate the desired number of etch cycles to remove 400 µm of silicon.
NOTE: This will vary depending on the tool that is utilized for the process, as they will influence the etch rate (in this case, it was 1.8 µm/cycle). - To prevent accidentally etching the Si3N4 layer, stop the dry etching at least 10 µm before (i.e. at 390 µm). Remove the remaining silicon using the traditional highly selective KOH at 80 °C for 5 min.
- Deposit 20 nm of Cr and 150 nm of Au metal layers consecutively using an E-beam evaporation process34 and a shadow mask to fabricate the sensing electrode in the T-MCC.
- At this stage, the T-MCCs in the wafer will be connected by tiny silicon holders. Separate the T-MCCs by carefully puncturing the tiny holders.
- Microfabrication steps for B-MCC (Figure 1B)
- On a clean thermally oxidized silicon substrate (400µm thick), deposit Cr/Au (20 nm, 150 nm) metal layers using a shadow mask, similar to step 1.1.9.
- Use standard photolithography32 with backside alignment, followed by a KOH wet-etching step to release the B-MCCs.
NOTE: The T- and the B-MCC have 2.5 x 1.5 cm contact pads.
2. Biofunctionalization of the capacitive sensor
NOTE: Briefly, this step is about coating the T-MCC electrode with Human Fibronectin (Fn) to facilitate platelet attachment onto the sensor.
- Prior to biomolecular coatings, clean the sensing electrode with oxygen plasma for 45 s at 100 W.
- Add 1-Dodecanethiol (1 mM) in 200-proof ethanol to the sample well on T-MCCs.
- Place the T-MCCs in a container filled with dry nitrogen, sealed, and wrapped with parafilm for 24-48 h.
- Rinse the gold surface with deionized water and 200-proof ethanol, followed by nitrogen drying at room temperature. At this stage, store the sensors at 2-8 °C in a dry nitrogen atmosphere until the next steps.
- Add Fn solution in phosphate-buffered saline (PBS, 50 µg/mL) to the well 12 h before the measurement and incubate at 37 °C for 2-8 h.
3. Capacitance sensor setup
NOTE: Figure 2 represents the photograph of the experimental setup.
- Use an LCR meter with micro positioners and needle probes to make electrical contact with the sensor.
- Employ 3D-printed plastic fixtures (see Table of Materials) to securely place the T- and the B-MCC. The bottom fixture is equipped with stoppers on the x-y axis to ensure precise alignment of the T-MCC over the B-MCC to form a capacitor.
NOTE: All 3D printers used were from the University of Washington. - Apply a sinusoidal signal (0.5 V) at 100 kHz and an 8 Hz sampling rate.
4. Preparation of citrated platelet-rich-plasma (c-PRP)
NOTE: All blood samples were from volunteers who participated in this research. None of the participants had a previously known platelet abnormality or clotting disorder, and they had not taken any platelet medications, including Non-Steroidal Anti-inflammatory Drugs (NSAIDs), in the two weeks leading up to sample collection. A licensed phlebotomist conducted a blood draw using a 21 G needle to standard 3.2% citrate tubes. The first 1 mL was discarded to avoid tissue factor contamination. Samples were transported in a polystyrene container, and all measurements were performed within 6 h of blood draw.
- Centrifuge the whole blood sample at 200 x g for 10 min at 20 °C to obtain c-PRP.
- Transfer the c-PRP to a sterile container and conduct the platelet count. For inhibitor studies, incubate the c-PRP with a predefined concentration of the drug in Tyrode's buffer (see Results section for details).
5. Platelet functional assay
NOTE: The schematic for the presented platelet functional assay is shown in Figure 3.
- Assemble the T- and the B-MCC in the 3D-printed fixtures.
- Measure the baseline capacitance measurement for 5 min, then add 45 µL of c-PRP to the sample well in the T-MCC.
- Wait for 30 min for the platelets to adhere to the Fn-coated electrode in the T-MCC.
- Remove 30 µL of the c-PRP carefully without touching the platelets attached to the membrane, and immediately replenish with Tyrode's buffer. Repeat washing at least 5 times with a 20 s interval to ensure that no additional platelets or macroaggregates land on the sensing electrode after activation.
- Add 10 µL of the agonist solution (Thrombin or ADP) at a desired concentration. Allow 80 min equilibration time.
6. Data and statistical analysis for the signal markers
NOTE: Capacitance is measured continuously from steps 5.2-5.5.
- For the capacitance signal during the adhesion phase (step 5.3), measure the maximum change in capacitance after the 30 min equilibration time, which is referred to as ΔCadh.
- Similarly, for the activation phase (step 5.5), measure the maximum change in capacitance, which is referred to as ΔCact, and the slope of the curve between 200-300 s after activation, which is referred to as Sact.
- Use analysis of variance (ANOVA) with Tukey's post-hoc to compare the results between the groups. Use Shapiro-Wilk Goodness of Fit for normal distribution analysis.
Wyniki
This study aims to conduct a dynamic assessment of platelet function. Following the protocol described above, the c-PRP solution was prepared, and platelets were seeded onto the Fn-coated electrode in T-MCC. The free-floating platelets were washed out by the washing step, and an agonist was added to activate the attached platelets. Detailed results and discussion can be found in our previous report28.
Figure 4A represents the results for a c-PRP solution with a predetermined platelet count (250 x 103 platelets µL-1). During the adhesion phase of the measurement (Figure 4B), the capacitance linearly decreased with time. After activating the attached platelets with thrombin (final concentration: 3.5 U mL-1), the capacitance reading exponentially decreased to a steady state (Figure 4C). Following the protocol described above for data analysis, three different signal marker names ΔCadh, ΔCact, and ΔSact were obtained.
Experiments were conducted on blood samples from at least 15 healthy volunteers to evaluate the sensitivity of this approach towards platelet activation levels, platelet counts, and antiplatelet drugs. Figure 5A,C represents the capacitance signal during the adhesion phase of the measurement for c-PRP samples modulated for a predefined platelet count (250, 100, 10, and 1 x 103 platelets µL-1; Figure 5A) and aspirin inhibition (0 mM, 0.1 mM, 0.51 mM, 1.3 mM; Figure 5C). As shown in Figure 5B, the signal marker ΔCadh has a high correlation with platelet count (F (3,16) = 46.23, p < 0.00001; one-way ANOVA). A statistically significant decrement in ΔCadh was observed only for a 1.3 mM aspirin dosage ((p = 0.0095, ANOVA post-hoc; Figure 5D). The rate of exponential decrease in capacitance following platelet stimulation increased proportionally with the platelet count (Figure 6A). The magnitude of capacitance decrease increased with increasing platelet count, which indicated a trend in ΔCact and platelet counts (F (3,16) = 92.37, p < 0.00001; one-way ANOVA; Figure 6B). The results also displayed a similar increasing trend in Sact and platelet count (F (3,16) = 43.62, p < 0.00001; one-way ANOVA, Figure 6C).
Experiments on blood samples incubated with a fixed concentration of aspirin revealed that the reduction in capacitance diminished as the aspirin dosage increased. (Figure 6D). ΔCact (F (3,16) = 123.0, p < 0.00001; one-way ANOVA; Figure 6E) and Sact (F (3,16) = 90.71, p < 0.00001; one-way ANOVA; Figure 6F) showed a decreasing trend with aspirin dosage). Between the high (1.3 mM) and medium (0.51 mM) dose aspirin data, a statistically significant difference was seen in Sact (p = 0.038; Figure 6E), but no significant difference was found in ΔCact (p = 0.43; Figure 6F). Figure 6G represents the capacitance signal for c-PRP samples activated with a predefined concentration of thrombin (0.1 mL-1, 3.5 mL-1, 10 U mL-1). Results displayed that the capacitance decrease after activation increased with higher thrombin concentration, and both ΔCact, Sact showed a statistically significant trend with thrombin concentration (Figure 6H,I). For 3.5 UmL-1 and 10 UmL-1 thrombin concentrations, the ΔCact and Sact increased by statistically insignificant increments (p≥ 0.29).
In summary, these results underscore the potential utility of the developed protocol in offering a dynamic assessment of the adhesion and activation functionality of platelets. Furthermore, these findings indicate that the sensor can be employed to investigate platelet physiology and the impacts of various inhibitors on platelet function. It also has the potential to furnish critical insights into platelet dysfunction, thereby informing the development of novel hemostatic or thrombotic strategies.

Figure 1: The membrane capacitance sensor (MCS) for platelet functional assay. (A,B) Microfabrication flowcharts for (A) the Top chip and (B) the Bottom chip of the capacitance sensor. (C) The pattern of electrodes for the Top chip and Bottom chip. Please click here to view a larger version of this figure.

Figure 2: Benchtop prototype of the platelet function analyzer. The center-right of the picture is the LCR meter for capacitance measurement. In front of it are the micro-positioners with needle probes. At the center is the MCS sensor inside a 3D-printed fixture. On the left is a laptop with the data acquisition program. Please click here to view a larger version of this figure.
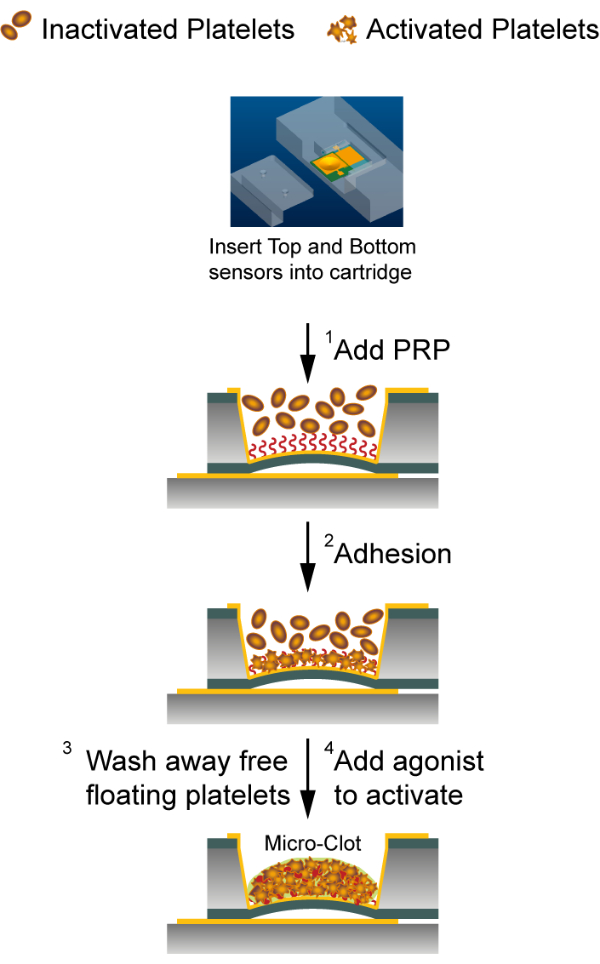
Figure 3: Platelet function evaluation assay with the capacitance sensor system. The bottom and the top chips were kept in a plastic cartridge for a fixed relative position. (1) Human platelet-rich plasma was added to the top chip. (2) Platelets attached to the fibronectin-coated sensing electrode. (3) Unbound platelets were removed by washing procedure. (4) Attached platelets were activated using thrombin or ADP. Please click here to view a larger version of this figure.

Figure 4: A representative output signal following the presented protocol. (A) Capacitance response in different functional phases for a human plasma sample activated using 3.5 U mL-1 of thrombin and with a predetermined platelet count of 250 x 103 platelets µL-1. (B) Sensor signal reading during the adhesion phase with the capacitance parameter ΔCadh, which is the maximum change in capacitance. (C) Sensor signal reading after activation with the capacitance parameters ΔCact and Sact. ΔCact was the maximum capacitance change, and Sact was the capacitance change over time, which was calculated as an average slope between 200 s and 250 s after adding the agonist. Please click here to view a larger version of this figure.

Figure 5: Impact of Platelet count and dysfunction in capacitance signal during the adhesion phase. (A) Sensor capacitance responses to PRP with different predefined platelet counts. (B) ΔCadh increases when platelet count increases. (C) Capacitance readings for PRP samples treated with various concentrations of aspirin. The platelet count in all samples was predefined to 250 x 103 platelets µL-1. (D) ΔCadh drops for an aspirin concentration of 1.3 mM (p = 0.0095, ANOVA post-hoc) For (A,C). The solid lines with shaded regions show the standard deviation. For (B,D), the line in the middle is the mean, and the bar is the standard deviation of data points. P-values were calculated from one-way ANOVA with Tukey's post-hoc. *p < 0.05, **p < 0.01 and NS denotes not significant. Please click here to view a larger version of this figure.
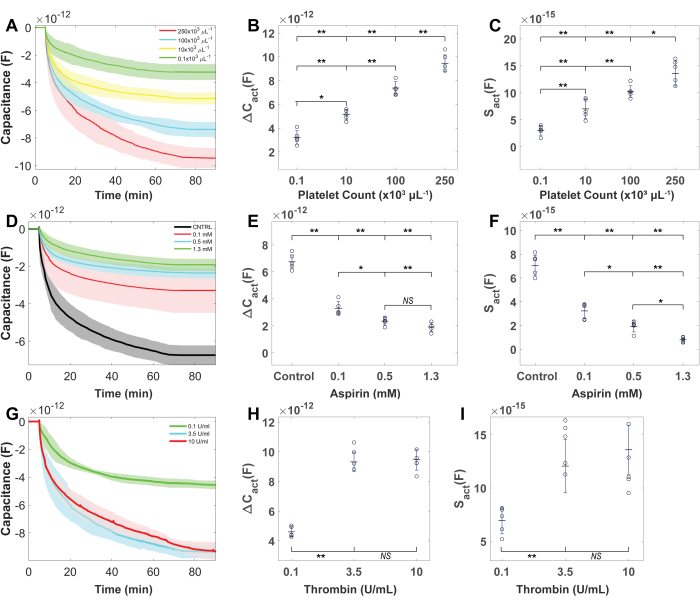
Figure 6: Platelet count, inhibitor, and sensor response activation levels after platelet activation. (A) The capacitance reading decreases after platelet activation increases with the platelet count. (B) ΔCact and (C) Sact increases when platelet count increases. (D) Capacitance decreases after activation reduces with increasing aspirin concentration. (E) ΔCact and (F) Sact have a dose-dependent decrement with aspirin concentration (TxA2 inhibition). (G) Sensor capacitance decrement increased with increasing concentrations of thrombin. (H) ΔCact and (I) Sact increase with increasing concentrations of thrombin. Between 3.5 U mL-1 and 10 U mL-1 of thrombin, no significant differences were observed in ΔCact and Sact. Platelet counts were maintained at 250 x 103 platelets µL-1. For (A,D,G), solid lines show the mean, and shaded regions show the standard deviation. For (B,C,E,F,H,I), the line in the middle is the mean, and the bar is the standard deviation of data points. P-values were calculated from one-way ANOVA with Tukey's post-hoc. *p < 0.05, **p < 0.01 and NS denotes not significant. Please click here to view a larger version of this figure.
Dyskusje
This study pioneered a novel capacitance-based method for assessing platelet function, which evaluates both adhesion and post-activation platelet dynamics within a single device, marking the first reported instance of such an approach. The novel experimental protocol introduces a relatively straightforward technique to counteract the impacts of fibrin formation and plasma clotting factors through a wash-out procedure. This results in measurements capable of discerning various factors that affect platelet function. Compared to traditional optical-based evaluation assay, this capacitance sensor holds promise for providing a more dynamic assessment of platelet function by integrating aspects of adhesion and aggregation. Importantly, it exhibits capabilities for detecting diverse platelet dysfunctions in a unique mode, setting it apart from existing assessment methods.
By employing microfabrication techniques, a standardized testing protocol, and comprehensive data analysis, the proposed sensor system enables dynamic multiparameter evaluation of platelet function, ensuring high accuracy, reliability, and stability. Simplified calibration and characterization processes are achieved, as calibration values from any randomly selected sensor can be universally applied to the entire batch of mass-produced devices. Additionally, chip design safeguards the bottom silicon chip from bio-sample contamination, rendering them reusable for multiple measurements and minimizing system disposables. A critical step in the platelet assay is the wash-out procedure (step 5.4), where 30 µL of the c-PRP is removed and replenished with platelet-free Tyrode's buffer. This step is important to ensure no additional platelets attach to the electrode or microclot after activation and to maintain this protocol's high sensitivity and specificity. However, the attached platelets can be activated if this step is not performed carefully. For example, variations in sample volume aspirated or excessive turbulence can cause shear stress-driven activation of platelets. To minimize these effects, it is essential to use consistent and gentle pipetting techniques, particularly in experiments. Various physiological blood parameters-such as ion concentrations, protein levels (e.g., tissue factor), and vitamins (e.g., vitamin K)-along with comorbidities like leukemia, myeloma, uremia, or the use of certain medications, can influence platelet function and may also complicate testing and interpretation35,36,37,38. In addition, measurements were conducted using citrated plasma in this pilot study to demonstrate the feasibility of a dynamic multiparameter platelet function assay. It remains to be seen whether the MCS sensor could offer an overall assessment of hemostatic function using whole-blood samples, and in the presence of various pathological conditions and biological variabilities.
A notable feature of the described protocol is its simplicity in exploring the interplay between physiological parameters and platelet function. The easy biofunctionalization of the sensor with matrix proteins on the Cr/Au-S3N4 membrane facilitates the examination of how various matrix ligands affect platelet adhesion and activation. By stimulating platelets adhered to immobilized agonists, such as collagen or TxA2, on the sensor membrane, diverse aspects of platelet function can be analyzed. This method allows for the investigation of how distinct physiological conditions or stimuli impact platelet behavior, offering valuable insights into platelet function across varied scenarios.
Ujawnienia
The authors declare no competing interests.
Podziękowania
The authors express their gratitude to Dr. Moritz Stolla and Dr. Jason Acker for their valuable discussions and technical assistance. They also acknowledge the Biology Imaging Facility at the University of Washington for its infrastructure and support. This work received partial funding from the CoMotion Innovation Fund at the University of Washington (Grant No. 682548, D.Y.G.).
Materiały
| Name | Company | Catalog Number | Comments |
| 1-Dodecanethiol | Sigma-Aldrich, MO, U.S.A | 471364-100ML | 1 mM |
| 200-proof ethanol | Sigma-Aldrich, MO, U.S.A | EX0276-1 | |
| 3D printer | Shenzhen Creality 3D Technology Co, Ltd. | Ender-3 V3 | |
| 3D printing material | HATCHBOX 3D, CA, U.S.A | 3D PLA-1KG-1.75 | |
| Adenosine 5′-diphosphate | Sigma Aldrich, U.S.A | 01905-250MG-F | ADP |
| Aspirin | Sigma-Aldrich, MO, U.S.A | A2093-100G | |
| Deep Reactive Ion Etching | Omega Engineering, Inc. | SPTS Rapier DRIE | |
| Dimethyl Sulfoxide | Sigma-Aldrich, MO, U.S.A | D8418-50ML | DMSO |
| High Vacuum Deposition Systems | CHA | SEC-600 | |
| Human Fibronectin | Sigma-Aldrich, MO, U.S.A | CLS356008-1EA | Fn |
| KOH | Sigma-Aldrich, MO, U.S.A | P1767-250G | |
| LCR meter | Keithley Instruments, Inc., OH, U.S.A | Keithley EL 4980AL | |
| LCR meter holders | Signatone Corporation, CA, U.S.A | SCA-50-4 | |
| Mask Aligner System | ABM, U.S.A, Inc. | ABM/6/350/NUV/DCCD/SA | |
| Micro-positioners | Signatone, CA, U.S.A | S-725 | |
| needle probe | Signatone Corporation, CA, U.S.A | SCAT5T-4 | 12.5 μm radius |
| Phosphate Buffered Saline | Sigma-Aldrich, MO, U.S.A | P4474-1L | PBS, pH 7.4 |
| Reactive Ion Etching | Plasma-Therm,U.S.A | RIE Vision 320 | |
| silicon substrate | Wafer World Inc | SKU# 1766 | |
| Standard 3.2% citrate tubes | Tiger Medical, NJ, U.S.A. | Covidien / Cardinal Health 8881340478 Monoject | |
| Thrombin | Enzyme Research Laboratories, U.S.A | HT 1002a | |
| Ticagrelor | Sigma-Aldrich, MO, U.S.A | PHR2788-400MG | |
| Tyrode’s buffer | Boston Bioproducts, U.S.A | BSS-375 | |
| UV photoresist | AZ electronic materials, NC, U.S.A. | AZ 9260 | 15um |
Odniesienia
- George, J. N. Platelets. Lancet. 355 (9214), 1531-1539 (2000).
- Wendelboe, A. M., Raskob, G. E. Global burden of thrombosis: Epidemiologic aspects. Circ Res. 118 (9), 1340-1347 (2016).
- Heit, J. A. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 12 (8), 464-474 (2015).
- Moran, A. E., et al. The global burden of ischemic heart disease in 1990 and 2010: The global burden of disease 2010 study. Circulation. 129 (14), 1493-1501 (2014).
- Campbell, B. C. V., et al. Ischaemic stroke. Nat Rev Dis Primers. 5 (1), 70 (2019).
- Ruggeri, Z. M. Platelets in atherothrombosis. Nat Med. 8 (11), 1227-1234 (2002).
- Harrison, P. Platelet function analysis. Blood Rev. 19 (2), 111-123 (2005).
- Van Der Meijden, P. E. J., Heemskerk, J. W. M. Platelet biology and functions: New concepts and clinical perspectives. Nat Rev Cardiol. 16 (3), 166-179 (2019).
- Bhatt, D. L., Topol, E. J. Scientific and therapeutic advances in antiplatelet therapy. Nat Rev Drug Discov. 2 (1), 15-28 (2003).
- Mcfadyen, J. D., Schaff, M., Peter, K. Current and future antiplatelet therapies: Emphasis on preserving hemostasis. Nat Rev Cardiol. 15 (3), 181-191 (2018).
- Michelson, A. D. Advances in antiplatelet therapy. Hematology Am Soc Hematol Educ Program. 2011, 62-69 (2011).
- Sweeny, J. M., Gorog, D. A., Fuster, V. Antiplatelet drug 'resistance'. Part 1: Mechanisms and clinical measurements. Nat Rev Cardiol. 6 (4), 273-282 (2009).
- Mega, J. L., Simon, T. Pharmacology of antithrombotic drugs: An assessment of oral antiplatelet and anticoagulant treatments. Lancet. 386 (9990), 281-291 (2015).
- Carr, M. E. Development of platelet contractile force as a research and clinical measure of platelet function. Cell Biochem Biophys. 38 (1), 55-78 (2003).
- Gresele, P., Bury, L., Mezzasoma, A. M., Falcinelli, E. Platelet function assays in diagnosis: An update. Expert Rev Hematol. 12 (1), 29-46 (2019).
- Pakala, R., Waksman, R. Currently available methods for platelet function analysis: Advantages and disadvantages. Cardiovasc Revasc Med. 12 (5), 312-322 (2011).
- Paniccia, R., Priora, R., Liotta, A. A., Abbate, R. Platelet function tests: A comparative review. Vasc Health Risk Manag. 11, 133-148 (2015).
- Rand, M. L., Leung, R., Packham, M. A. Platelet function assays. Transfus Apher Sci. 28 (3), 307-317 (2003).
- Alafeef, M., Dighe, K., Moitra, P., Pan, D. Rapid, ultrasensitive, and quantitative detection of sars-cov-2 using antisense oligonucleotides directed electrochemical biosensor chip. ACS Nano. 14 (12), 17028-17045 (2020).
- Chowdhury, A. D., Takemura, K., Li, T. C., Suzuki, T., Park, E. Y. Electrical pulse-induced electrochemical biosensor for hepatitis e virus detection. Nat Commun. 10 (1), 3737 (2019).
- Park, J. H., et al. Capacitive biosensor based on vertically paired electrodes for the detection of sars-cov-2. Biosens Bioelectron. 202, 113975 (2022).
- Qureshi, A., Pandey, A., Chouhan, R. S., Gurbuz, Y., Niazi, J. H. Whole-cell based label-free capacitive biosensor for rapid nanosize-dependent toxicity detection. Biosens Bioelectron. 67, 100-106 (2015).
- Rassaei, L., Mathwig, K., Kang, S., Heering, H. A., Lemay, S. G. Integrated biodetection in a nanofluidic device. ACS Nano. 8 (8), 8278-8284 (2014).
- Wang, L., et al. A sensitive DNA capacitive biosensor using interdigitated electrodes. Biosens Bioelectron. 87, 646-653 (2017).
- Zhurauski, P., et al. Sensitive and selective affimer-functionalized interdigitated electrode-based capacitive biosensor for her4 protein tumor biomarker detection. Biosens Bioelectron. 108, 1-8 (2018).
- Pourang, S., et al. Assessment of fibrinolytic status in whole blood using a dielectric coagulometry microsensor. Biosens Bioelectron. 210, 114299 (2022).
- Sekar, P. K., et al. Simultaneous multiparameter whole blood hemostasis assessment using a carbon nanotube-paper composite capacitance sensor. Biosens Bioelectron. 197, 113786 (2022).
- P, K. S., et al. Comprehensive multiparameter evaluation of platelet function using a highly sensitive membrane capacitance sensor. Biosens Bioelectron. 228, 115192 (2023).
- Franssila, S. . Introduction to microfabrication. , (2010).
- Syed Rizvi, S. R. . Handbook of photomask manufacturing technology. , (2018).
- Yota, J., Hander, J., Saleh, A. A. A comparative study on inductively-coupled plasma high-density plasma, plasma-enhanced, and low pressure chemical vapor deposition silicon nitride films. J Vacuum Sci Technol A. 18 (2), 372-376 (2000).
- Thompson, L. F. An introduction to lithography. ACS Symp Series. 219, 1-13 (1983).
- Laermer, F., Urban, A. Challenges, developments and applications of silicon deep reactive ion etching. Microelectron Eng. 67 - 8, 349-355 (2003).
- Harsha, K. S. . Principles of vapor deposition of thin films. , (2005).
- Lea-Henry, T. N., Carland, J. E., Stocker, S. L., Sevastos, J., Roberts, D. M. Clinical pharmacokinetics in kidney disease: Fundamental principles. Clin J Am Soc Nephrol. 13 (7), 1085-1095 (2018).
- Offermanns, S. Activation of platelet function through g protein-coupled receptors. Circ Res. 99 (12), 1293-1304 (2006).
- Trejo-Velasco, B., et al. Impact of comorbidities and antiplatelet regimen on platelet reactivity levels in patients undergoing transcatheter aortic valve implantation. J Cardiovasc Pharmacol. 78 (3), 463-473 (2021).
- Violi, F., Pignatelli, P., Basili, S. Nutrition, supplements, and vitamins in platelet function and bleeding. Circulation. 121 (8), 1033-1044 (2010).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone