Method Article
Measuring Cardiac Output in a Swine Model
En este artículo
Resumen
Thermodilution, pressure-volume loop catheters, and contrast ventriculography are reliable and accurate methods for determining cardiac physiology such as stroke volume and cardiac output in a laboratory setting in swine.
Resumen
Swine are frequently used in medical research given their similar cardiac physiology to that of humans. Measuring cardiac parameters such as stroke volume and cardiac output are essential in this type of research. Contrast ventriculography, thermodilution, and pressure-volume loop (PV-loop) catheters can be used to accurately obtain cardiac performance data depending on which resources and expertise are available. For this study,five Yorkshire swine were anesthetized and intubated. Central venous and arterial access was obtained to place the necessary measurement instruments.A temperature probe was placed in the aortic root. A cold saline bolus was delivered to the right atrium and temperature deflection curve was recorded. Integration of the area under the curve allowed for the calculation of the current cardiac output.A pigtail catheter was percutaneously placed in the left ventricle and 30 mL of iodinated contrast was power injected over 2 seconds. Digital subtraction angiography images were uploaded to volumetric analysis software to calculate the stroke volume and cardiac output. A pressure volume-loop catheter was placed into the left ventricle (LV) and provided continuous pressure and volume data of the LV, which allowed the calculation of both stroke volume and cardiac output.All three methods demonstrated good correlation with each other. The PV-loop catheter and thermodilution exhibited the best correlation with a 3% error and a Pearson coefficient of 0.99, with 95% CI=0.97 to 1.1, (p=0.002). The PV-loop catheter against ventriculography also showed good correlation with a 6% error and a Pearson coefficient of 0.95, 95% CI=0.96 to 1.1 (p=0.01). Finally, thermodilution against ventriculography had a 2% error with r=0.95, 95% CI=0.93 to 1.11, (p=0.01). In conclusion, we state that the PV-loop catheter, contrast ventriculography, and thermodilution each offer certain advantages depending on the researcher's requirements. Each method is reliable and accurate for measuring various cardiac parameters in swine such as the stroke volume and cardiac output.
Introducción
Swine are frequently used in hemorrhage control and resuscitation research due to their similar physiology to humans. Integral to resuscitation research is continuous cardiac output monitoring to assess the physiological response to interventions. Several clinical systems exist such as pulmonary artery (PA) catheters and pulse contour analysis-based systems1. Additionally, echocardiography (echo), computed tomography (CT) and magnetic resonance imaging (MRI) can all be used to capture hemodynamic data. Images obtained during end-diastole and systole can be used to determine the volume of blood ejected during that cardiac cycle. While these techniques are minimally invasive, they only present data acquired at the time of imaging and do not provide continuous measures2. They are also either largely operator dependent (echo) or require advanced, expensive equipment (CT and MRI). Given different laboratories' capabilities and resources, there are various alternative methods to measure the cardiac output optimally in each instance.
Thermodilution is a common method of measuring cardiac output in the clinical setting using a Swan-Ganz catheter3. This method can be recreated in a laboratory setting in swine to directly measure the cardiac output. Contrast ventriculography can also be utilized if the fluoroscopic capability is readily available4. Finally, pressure-volume loop catheters offer a means of directly measuring the ventricular pressure and volume on a beat-to-beat basis and can generate more nuanced data5. This method utilizes electrical admittance and Wei's equation to measure the chamber volume. Compared to older conductance-based catheters, admittance catheters eliminate the parallel conductance phenomenon between the blood and the cardiac muscle, thereby producing more accurate measurements without requiring repeated calibration6.
The aim of this study is to validate the accuracy of these three methods against each other in terms of measuring cardiac stroke volume and output in a healthy swine model. Ultimately, each investigator can choose which approach suits their needs the best, depending on their study requirements and what resources are available to them.
Protocolo
Procedures were approved by the University of Maryland, Baltimore Institutional Animal Care and Use Committee (Approval #0320017) and conformed to National Institutes of Health guidelines for ethical animal research. Five adult male Yorkshire swine weighing between 50 and 70 kg were enrolled into the study. This study utilized a digital data collection system and paired software to record all hemodynamic and temperature data. Measuring cardiac parameters in the swine model consisted of the following steps: preparation, thermodilution, ventriculography, PV-loop catheter insertion, and finally euthanasia. All five animals underwent each of the three cardiac output measuring protocols.
1. Animal selection and housing
- Use adolescent male Yorkshire swine (Sus Scrofa) weighing 50-70 kg.
- House animals in caging at least 30 square feet in area with high-carbon bedding such as hay, straw, or pine shavings. House the animal individually, the night prior to the procedure.
- Allow acclimation period for new animals as per institution guidelines.
NOTE: This is typically 48-72 h for large mammals7. - Feed animals a standard diet and provide free access to water until the night before the experiment.
- Fast the animals the night before the procedure to minimize aspiration risk during endotracheal intubation.
- Monitor animals' health weekly by inspecting the skin for signs of injury such as scabs, scrapes, or abrasions. Ensure normal work of breathing (15-30 breaths/min) and proper, interactive behavior. Ensure that the oral mucosa is pink, moist, and without discharge.
- Weigh animals regularly to confirm that they have adequate nutrition. Report any abnormalities to the veterinary staff and then exclude the animal from the protocol.
2. Sedation and induction of general anesthesia
- Sedate the animal in its housing area by intramuscular injection of Telazol (4-5 mg/kg)/ Xylazine (1.8-2.2 mg/kg) in the fat pad caudal to the ear.
- Wait until the animal is fully sedated and there is minimal to no response to stimulation to ensure safe handling and transport of the animal.
- Transport the animal from the housing area to the procedure room and place it in dorsal recumbency on the operating table.
- Place a pulse oximetry probe on the animal's ear and begin ventilating the animal with a snout mask using a mechanical ventilator with 100% O2. Ensure proper seal of rubber gasket of the mask around the snout. Once a proper seal is ensured, administer 3-4% isoflurane until general anesthesia is induced and the jaw is relaxed.
NOTE: Be sure to follow institutional guidelines for inhaled volatile agent use. In general, the procedure room must be well ventilated, and a proper scavenging/ventilation mechanism must be used to eliminate inhalation exposure. - Place an orotracheal tube using a laryngoscope by turning off the isoflurane vaporizer and removing the snout mask. Have a second person hold the jaws open while the operator inserts the laryngoscope and displaces the epiglottis ventrally away from the soft palate. Once the vocal cords are visualized, insert an 8-0 endotracheal (ET) tube through vocal cords by at least 5 cm.
- Inflate the ET cuff with 10 mL of air and secure the ET tube to the animal's snout using umbilical tape. Confirm the tube placement by the chest rise, end-tidal CO2, and/or chest auscultation.
- Connect the ET tube to the anesthetic machine with a heat and moisture exchanger.
- Adjust the mechanical ventilator settings to deliver an inspired O2 fraction of 30%, with a tidal volume 7-10 mL/kg, and a respiratory rate of 10-16 breaths/min, to maintain an end-tidal CO2 tension of 38-42 mmHg.
- Return and maintain inhaled anesthetic using 1.5-3% isoflurane. Monitor the animal for signs of pain and discomfort such as involuntary movements or tachycardia. Adjust isoflurane until movements extinguish or tachycardia resolves.
3. Surgical site sterilization and preparation
- Clip the hair overlying and percutaneous access sites (bilateral ventral neck) by using an electric hair clipper.
- Prepare and scrub all percutaneous puncture sites with betadine and isopropyl alcohol and allow to dry completely.
- Place sterile drapes around the operative sites to preserve the sterile surgical fields and prevent contamination. Secure these in place with staples.
- Secure the animal to the operating table by restraining forelimbs and hindlimbs to the table using either tape or rope. Place the heating pad underneath the animal and set to 37 °C.
- Apply a water-based lubricant to the tip of the temperature probe and insert the probe into the rectum to provide continuous body temperature data.
- Place the ECG adhesive electrodes on the right and left lateral chest wall. Attach the ECG leads to the adhesive electrodes and connect ECG leads to the data collection unit.
- Carefully, flip the animal to ventral recumbency, ensuring that the airway tubing and ECG leads are controlled during transfer.
4. External jugular vein cannulation
NOTE: Jugular venous access is obtained for the right atrial venous cannula insertion during the thermodilution procedure.
- Use ultrasound (US) guidance to locate the external jugular vein in the jugular furrow located in the lateral neck region. Puncture the skin with an 18 G needle placed at a 45° angle to the skin and advance the tip into the venous lumen under US guidance.
- Pass an 0.035" Seldinger guidewire through the needle and into the venous lumen. Remove the needle while leaving the guidewire in place within the venous lumen.
- Make a 5 mm skin incision adjacent to the wire using a #11 blade scalpel, and thread a 15 cm, 7 Fr sheath with a dilator into the vein over the guidewire. Remove the guidewire and the dilator. Ensure that the sheath remains in position. Stitch the sheath in place by suturing it to the skin with 3-0 silk sutures.
- Repeat steps 4.1 through 4.3 to cannulate the contralateral external jugular vein.
5. Carotid artery cannulation
NOTE: The carotid artery cannulation is performed to provide access to the LV and aortic root during thermodilution, contrast ventriculography, and PV-loop catheter insertion.
- Locate the carotid artery lateral to the trachea using an US probe. Ensure pulsatile flow using color Doppler imaging if available.
- Puncture the skin with an 18 G needle placed at a 45° angle to the skin and advance it into the arterial lumen under US vision. Advance an 0.035" Seldinger guidewire through the needle and into the arterial lumen. Remove the needle while leaving the guidewire in position within the arterial lumen.
- Make a 5 mm skin incision adjacent to the wire with a #11 blade scalpel, and thread a 20 cm 7 Fr sheath with a dilator into the artery over the guidewire. Leave 5-10 cm of the sheath outside of the skin. Remove the guidewire and the dilator and ensure that the sheath remains in position. Secure the sheath in place by suturing it to the skin with 3-0 silk sutures8.
6. Cardiac output measurement
NOTE: All of the following methods are performed sequentially in each of the 5 animals used in this study.
- Thermodilution
- Insert a T-type thermocouple probe via the carotid arterial sheath and guide the probe into the aortic root using fluoroscopic guidance.
- Attach the probe to the data collection system. Allow several minutes of data collection to establish a confident baseline aortic temperature. A confident baseline temperature is achieved when the temperature stays within 1 °C of the central value over 2-3 min of data collection.
- Next, insert a 5 Fr, 110 cm catheter via the external jugular vein sheath and navigate the catheter to the right atrium using fluoroscopy.
- Once the position is verified, forcefully flush 20 mL of 12 °C saline into the catheter.
- Observe the temperature deflection curve on the data collection software. Highlight this region and use the cardiac output function of the software to calculate cardiac output (Figure 1).
- Repeat this process 3-5 times as needed to obtain an average value across these measurements.
- Ventriculography
- Using portable x-ray fluoroscopic guidance, insert a 0.035" guidewire via the carotid arterial sheath and into the left ventricle. Advance the sheath so that the tip traverses the aortic valve. Remove the 0.035" guide wire and insert an 80-cm marker pigtail catheter via the carotid arterial sheath so that the pigtail is resting in the LV apex. Withdraw the sheath 5 cm while leaving the pigtail in place so that the sheath is no longer within the LV.
- Ensure that all personnel in the room are wearing 0.5 mm lead apron equivalents. Have anyone within 1 m of the emitter wear leaded glasses.
- Minimize the beam-on time and ensure that the emitter is well collimated to reduce exposure to lab personnel.
- Ensure that all personnel stand as far away from emitter as possible while still being able to perform the procedure in order to reduce exposure.
- Attach the catheter to a contrast power injector with at least 30 mL of iodinated contrast loaded. Note the current heart rate of the animal.
- Configure the power injector to deliver 15 mL/s of contrast for a total of 30 mL and arm the device.
- Configure the fluoroscope for digital subtraction angiography (DSA) at 30 frames/s at 30° left anterior obliquity.
- Start DSA on the fluoroscope and wait for the image to subtract. Begin the power injection as soon as the subtraction image is shown by the fluoroscopy machine. Do this by pressing the injector button as soon as the fluoroscopy machine subtracts the radio-opaque material from the image so that only the LV chamber is opacified during the image series (Figure 2A).
NOTE: Alternatively, classical fluoroscopy can be used for ventriculography if DSA is unavailable. - Upload these images to a picture archiving and communication system (PACS).
- Import the ventriculogram images into a quantitative imaging software.
- Using the left ventricular analysis function, calibrate the software to the image using the 1 cm marks on the marker pigtail as a reference.
- Click on the End Diastole button and search the DSA images for the frame depicting the largest LV volume indicating end-diastole. Then, trace the LV border using the mouse in small increments to ensure accuracy.
- Next click on the End Systole button and search for the frame depicting end systole where LV volume is the least. Again, trace the LV border in the same manner as the previous step.
- Click the Analyze button. The program then performs quantitative volumetric analysis using the Dodge-Sandler area-length method9 to calculate end-systolic volume, end-diastolic volume, as well as stroke-volume. The data is then output in an LV analysis document.
- Determine the cardiac output by multiplying the measured stroke volume by the previously recorded heart rate.
- Using portable x-ray fluoroscopic guidance, insert a 0.035" guidewire via the carotid arterial sheath and into the left ventricle. Advance the sheath so that the tip traverses the aortic valve. Remove the 0.035" guide wire and insert an 80-cm marker pigtail catheter via the carotid arterial sheath so that the pigtail is resting in the LV apex. Withdraw the sheath 5 cm while leaving the pigtail in place so that the sheath is no longer within the LV.
- Pressure-volume loop catheter
- Presoak the PV-loop catheter in normal saline for at least 20 min prior to the use. Connect the catheter to the data collection system.
- Insert the tip of the PV-loop catheter containing the pressure transducer into a syringe of saline and hold the pressure transducer just underneath the meniscus. Using the coarse and fine adjustment buttons on the catheter module, adjust the output pressure signal until it reads 0 mmHg on the data collection software.
- Calibrate the PV-loop catheter blood resistivity and stroke volume per the manufacturer's instructions.
NOTE: The manufacturer suggests using a blood resistivity of 1.5 mΩ and a stroke volume of 60 mL for large animal models. - As in step 6.2.1, insert a 0.035" guidewire into the carotid arterial sheath and advance it into the LV using fluoroscopic guidance. Advance the sheath until it traverses the aortic valve. Remove the guidewire leaving the sheath in place and insert the PV-loop catheter under fluoroscopic guidance via the carotid arterial sheath until the pigtail portion is resting in the LV apex (Figure 2B). Withdraw the sheath ~5 cm so that it is no longer within the LV while leaving the pigtail in place.
- Per manufacturer instructions, perform a baseline scan prior to collecting data by navigating to the Baseline scan option on the data acquisition screen and press the Enter button. A baseline scan is taken using the PV-loop catheter system to confirm accurate heart rate measurement. Press the Enter button again to begin pressure and volume data acquisition. The volume segments can be scrolled through to acquire the best volume waveform.
- Check the pressure volume curve being output to the data collection software. Ensure that the PV-loop is recorded adequately, which should be rectangular with smooth edges. (Figure 2C). If not, gently reposition the catheter either by twisting, or moving back and forth until an adequate loop is recorded. Calculate stroke volume and cardiac output by multiplying the heart rate by the difference between the end-diastolic and end-systolic volume.
- Secure the catheter in place either with tape or suture to ensure adequate positioning throughout.
7. Euthanasia
- Euthanize the animal using >2 mEq/kg potassium chloride (50-70 mL of 2 mEq/mL KCl) injection via the jugular vein sheath.
- Continue general anesthesia and cardiac monitoring until ECG tracing shows no cardiac electrical activity and end-tidal CO2 tension reaches 0 mmHg.
NOTE: Hemodynamic monitoring was maintained throughout the experiment.
Resultados
The weight of the swine ranged from 51.4 kg to 61.5 kg with a mean weight of 56.6 ± 3.6 kg. The average stroke volumes measured by PV-loop catheter, ventriculography, and thermodilution across all five subjects were 58.0 ± 12.0 mL, 57.6 ± 8.5 mL, and 53.0 ± 9.8 mL, respectively. The average cardiac outputs measured by a PV-loop catheter, ventriculography, and thermodilution across all five subjects were 5.0 ± 1.1 L/min, 5.3 ± 1.2 L/min, and 5.2 ± 1.0 L/min, respectively (Table 1). Figure 3 shows the scatter plots of cardiac output measurements by each of the three methods compared with the other two. The line of identity demonstrates where all points would fall if there was perfect agreement between each test. The line of identity for all three methods falls within the 95% confidence interval range. When measuring cardiac output, the PV-loop catheter compared to thermodilution demonstrated the best correlation with a Pearson coefficient of r=0.99 (Figure 3A). The slope of the regression line was 1.03 indicating a 3% error with 95% CI=0.97 to 1.1, p=0.002. As seen in Figure 3B, the PV-loop catheter compared against ventriculography also exhibited good correlation with a Pearson coefficient r=0.95 The slope of the regression line was 1.06 indicating a 6% error with 95% CI=0.96 to 1.1, p=0.01. Lastly, ventriculography compared to thermodilution demonstrated agreement as well with a Pearson coefficient of r=0.93 (Figure 3C). The slope of the regression line was 1.02, indicating a 2% error with 95% CI=0.93 to 1.1 and p=0.01.
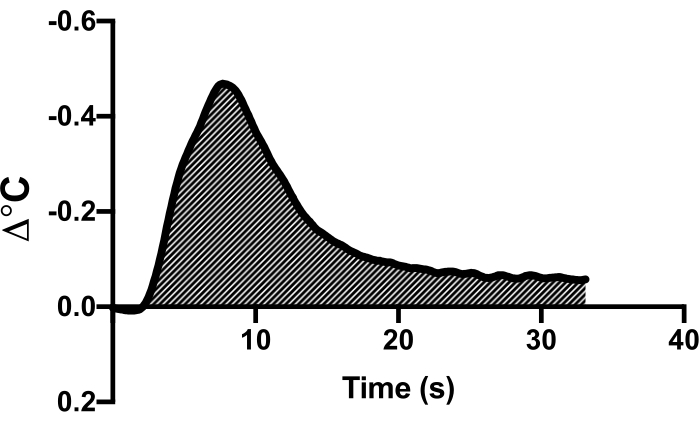
Figure 1: Example of thermodilution curve. An example thermodilution curve in a single representative animal demonstrating the deflection of temperature curve as a cold saline bolus is delivered. The area under the curve is used to calculate the cardiac output. Please click here to view a larger version of this figure.

Figure 2: Ventriculogram and PV Loops. (A) Left ventriculogram. (B) Successful PV-loop catheter placement in the apex of the LV. (C) Example of high-quality PV loops. Please click here to view a larger version of this figure.
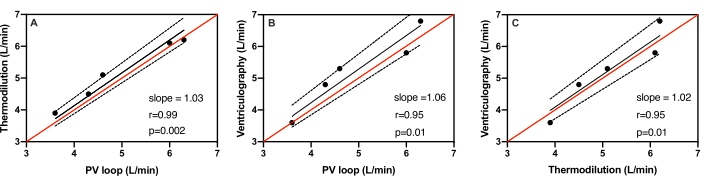
Figure 3: Linear regression of cardiac output measurements. Linear regression of cardiac output measurements in five different animals using (A) PV-loop catheter versus. thermodilution, (B) PV-loop catheter versus ventriculography, and (C) thermodilution versus ventriculography. The line of identity (red) falls within the 95% confidence range (dotted) of the best-fit-line (black) among all three methods. Please click here to view a larger version of this figure.
| Method | Stroke Volume (mL) | Cardiac Output (L/min) |
| PV-loop | 58.0 ± 12.0 | 5.0 ± 1.1 |
| Contrast Ventriculography | 57.6 ± 8.5 | 5.3 ± 1.2 |
| Thermodilution | 53.0 ± 9.8 | 5.2 ± 1.0 |
Table 1: Average results of cardiac parameters using PV loop, contrast ventriculography, and thermodilution.
Discusión
This study details a standardized method of three different ways to accurately measure cardiac output in swine. Swine has analogous cardiovascular anatomy and physiology to humans and is commonly used as a model for human cardiac physiology, specifically for pre-clinical evaluations of surgical and interventional processes10. This allows swine to serve as the primary model for cardiovascular physiology, pathology, and emerging biotechnology11. In order to assess these concepts, hemodynamic monitoring must be an accessible and reliable tool for swine models.
PV-loop catheters are not commonly used in human medicine due to inherent risks of prolonged ventricular cannulation (i.e., arrhythmia, thrombus formation, and myocardial injury). Despite this, they are occasionally used for short durations during cardiac catheterization procedures to measure stroke volume and stroke work13. However, in healthy non-survival animal studies, the benefit and versatility of PV-loop catheters generally outweigh the aforementioned risks. Other clinical tools include pulmonary artery catheters. However, this technique assumes the right heart output matches the left heart output and ignores beat-to-beat variations in output between the two chambers12. The thermodilution method described in this study mitigates these variations by measuring thermodilution across both ventricles of the heart simultaneously rather than just the right ventricle14. Given these limitations, contrast ventriculography, PV-loop catheter, or thermodilution can be used to reliably obtain cardiac performance data in the laboratory setting.
In terms of vascular cannulation, this study employed the Seldinger technique rather than a vascular cutdown. The Seldinger technique was used due to its rapid vascular access with novice level skill and few complications. Conversely, open vascular dissection requires a larger skillset and carries larger risk for complications that can either delay or even prohibit further experiment on the animal15. Additionally, this study utilizes carotid cannulation rather than femoral cannulation. This is largely due to the ease of accessing the heart chambers precluding the need to navigate the aortic arch for LV access. These protocol choices allowed for more efficient vascular access and endovascular navigation that give these methods versatility and ease of use in various protocols in the laboratory setting.
This study demonstrates that in healthy swine, there is a good correlation of cardiac parameters among different measurement methods including contrast ventriculography with quantitative volumetric analysis, thermodilution, and a PV-loop catheter. When measurement data between two methods are compared directly, the line of identity indicates where all the measurements would fall if perfect agreement existed between the two methods16. The PV-loop catheter, ventriculography, and thermodilution each produced measurements where the line of identity falls within the confidence interval range, which indicates accurate measurements by each method when compared to each other.
Based upon which resources and expertise are available, the optimal method can be chosen. If there is an access to a suite of measurement probes and data acquisition, PV-loop catheters or thermodilution are ideal choices depending on the protocol needs. PV-loop catheters offer real time, continuous data acquisition throughout the experimental protocol and provides a myriad of cardiac parameters including stroke volume, ejection fraction, end-diastolic volume, end-systolic volume, and stroke work. Thermodilution offers quick and easy, snap-shot measurements whenever needed, and avoids the need for ventricular cannulation. The method described in this study also has the advantage of measuring cardiac output across both ventricles of the heart. Finally, if there is a limited access to advanced measurement tools contrast ventriculography with volumetric analysis software is readily available and require comparatively little expertise to employ.
There are several limitations to each of these methods. Namely, they will all require readily available fluoroscopy to confirm various catheter positions when obtaining measurements. Choice of anesthetic will also influence cardiac performance, but each method will provide consistent measurements as long as the same anesthetic is used. Finally, this study is targeted towards healthy animals without cardiac pathology. The agreement and accuracy amongst these methods may diverge given different chronic disease states such as heart failure, valvular regurgitation, or cardiomyopathy. Nevertheless, these three methods offer researchers flexibility in choosing whichever method is the most ideal for a given experiment while providing reliable and accurate measurements of cardiac performance in healthy swine specimens.
Divulgaciones
The authors declare that there is no conflict of interest.
Agradecimientos
None
Materiales
| Name | Company | Catalog Number | Comments |
| 0.9% sodium chloride injection | Hospira | 0409-4888-50 | |
| 7 Fr Introducer Kit | Terumo | RCFW-5.0-35 | |
| Anesthesia Machine | Drager | Fabius Tiro | |
| Contrast Power Injector | GEHealthcare | E8004N | |
| Fluoroscope | GEHealthcare | OEC 9800 | |
| Heating/Cooling T/pump | Gaymar | Tp-700 | |
| Isoflurane | Baxter | 10019-360-40 | |
| Jackie catheter | Terumo | 40-5023 | |
| Omnipaque | GEHealthcare | 559289 | |
| PowerChart | ADinstruments | ML866/P | Software |
| PowerLab | ADinstruments | PL3516 | |
| PV-loop catheter | Transonic | Prefer pigtail tip to straight tip | |
| PV-loop module | Transonic | FFS-097-A004 | |
| Surgical suture, black braided silk, 3.0 | Surgical Speciaties Corp. | ||
| Thermocouple probe | ADinstruments | MLT1401 | |
| Ultrasound probe | Philips | L12-4 | |
| Various-sized syringes | |||
| ViewPlus | Sanders Data Systems | Software |
Referencias
- Pinsky, M. R., Payen, D. Functional hemodynamic monitoring. Critical Care. 9, 566-572 (2005).
- Geerts, B. F., Aarts, L. P., Jansen, J. R. Methods in pharmacology: Measurement of cardiac output. British Journal of Clinical Pharmacology. 71, 316-330 (2011).
- Argueta, E. E., Paniagua, D. Thermodilution cardiac output: A concept over 250 years in the making. Cardiology in Review. 27, 138-144 (2019).
- Higgins, C. B., et al. Quantitation of left ventricular dimensions and function by digital video subtraction angiography. Radiology. 144, 461-469 (1982).
- Abraham, D., Mao, L. Cardiac pressure-volume loop analysis using conductance catheters in Mice. Journal of Visual Experiments. (103), e52942 (2015).
- Porterfield, J. E., et al. Dynamic correction for parallel conductance, GP, and gain factor, α, in invasive murine left ventricular volume measurements. Journal of Applied Physiology. 107, 1693-1703 (2009).
- Obernier, J. A., Baldwin, R. L. Establishing an appropriate period of acclimatization following transportation of laboratory animals. Institution of Laboratory Animal Research Journal. 47, 364-369 (2006).
- Madurska, M. J., et al. Development of a selective aortic arch perfusion system in a porcine model of exsanguination cardiac arrest. Journal of Visualized Experiments. (162), e61573 (2020).
- Dodge, H. T., Sandler, H., Ballew, D. W., Lord, J. D. The use of biplane angiocardigraphy for the measurement of left ventricular volume in man. American Heart Journal. 60, 762-776 (1960).
- Crick, S. J., Sheppard, M. N., Ho, S. Y., Gebstein, L., Anderson, R. H. Anatomy of the pig heart: comparisons with normal human cardiac structure. Journal of Anatomy. 193, 105-119 (1998).
- Smith, A. C., Swindle, M. M. Preparation of Swine for the Laboratory. Institution of Laboratory Animal Research Journal. 47, 358-363 (2006).
- Franklin, D. L., Van Citters, R. L., Rushmer, R. F. Balance between right and left ventricular output. Circulation Research. 10, 17-26 (1962).
- Borlaug, B. A., Kass, D. A. Invasive hemodynamic assessment in heart failure. Cardiology Clinics. 29, 269-280 (2011).
- Thrush, D., Downs, J. B., Smith, R. A. Continuous thermodilution cardiac output: Agreement with fick and bolus thermodilution methods. Journal of Cardiothoracic and Vascular Anesthesia. 9, 399-404 (1995).
- Izer, J., Wilson, R., Hernon, K., Ündar, A. Ultrasound-guided vessel catheterization in adult Yorkshire cross-bred pigs. Veterinary Anesthesia and Analgesia. 44, 133-137 (2017).
- Hanneman, S. K. Design, analysis, and interpretation of method-comparison studies. AACN Advanced Critical Care. 19, 223-234 (2008).
Reimpresiones y Permisos
Solicitar permiso para reutilizar el texto o las figuras de este JoVE artículos
Solicitar permisoThis article has been published
Video Coming Soon
ACERCA DE JoVE
Copyright © 2025 MyJoVE Corporation. Todos los derechos reservados