Method Article
Mouse Model of Coronary Collateral Growth Through Repetitive Ischemia
摘要
This paper presents a method to study postnatal coronary collateral growth induced by repetitive ischemia in mice, including the surgical implantation of a pneumatic occluder on the left anterior descending artery, an automated inflation system for the repetitive ischemia protocol, and potential methods to evaluate collateral growth.
摘要
Coronary collaterals are a natural bypass in ischemic heart diseases (IHD), and so for many years, coronary collateral growth (CCG) has been a promising therapeutic target for IHD, particularly in patients with type 2 diabetes or metabolic syndrome in which CCG is impaired. However, this process is understudied, partly due to the lack of mouse models of CCG, even though other animal models, such as pigs, dogs, and rats, have been established. A mouse model can take advantage of the many genetic modifications available for the species, including lineage tracing and gene regulation (overexpression or knockout), to elucidate the process and mechanism of CCG, including the pathways and cell types involved. We, therefore, set out to develop a mouse model of CCG induced by repetitive ischemia (RI) via transient, repetitive occlusion of the left anterior descending artery (LAD). This manuscript provides details of this mouse CCG model, including the RI surgery to implant a pneumatic occluder on the LAD, the automated pressure-based inflation system used for controlling the pressure and timing of inflation, and the sequence of the RI protocol. This method has already generated one publication to elucidate the process of CCG induced by RI, showing that sprouting angiogenesis gives rise to mature coronary arteries in CCG in adult mouse hearts.
引言
Ischemic heart disease (IHD) is the leading cause of mortality in the United States, and more than 200,000 coronary artery bypass surgeries are performed annually in an effort to treat the disease1. Coronary collaterals, anastomoses between branches of the coronary arterial tree, are a natural bypass that can resupply blood to ischemic tissue downstream of a blockage2; however, people exhibit a wide variation in the extent of their native collateral networks3,4. Patients with IHD who have more extensive coronary collateralization have better outcomes during cardiac events, including reduced infarct size and mortality. Hence, coronary collateral growth (CCG) has been a therapeutic target for over a decade5,6,7. It is of particular interest for the growing number of patients with metabolic syndrome8, who exhibit poorer coronary collateralization9. However, until the process and mechanism of CCG are better understood, attempting to induce CCG for the treatment of IHD is unlikely to be fruitful.
Coronary collaterals have been studied in large animal models, and brief, repetitive occlusions of main coronary arteries have been used to induce CCG in pigs10, dogs11, and rats12. A mouse model of CCG, however, would have more advantages in studying the molecular and cellular mechanisms of CCG because of the many genetically modified mouse lines readily available, including lineage tracing, gene-specific or cell-specific transgenic and knockout lines. Interestingly, unlike humans, mice are reported to have no native coronary collaterals13,14, making them an attractive model to study coronary collateral formation. Indeed, a recent report showed that in patients with obstructive artery disease, nearly half (47%) had no collateralization (Rentrop grade 0)3; thus, a mouse model of CCG could be clinically relevant for patients with minimal native collateralization.
We, therefore, developed a mouse model of CCG induced by repetitive ischemia, with an inflatable balloon occluder over the left anterior descending artery (LAD) that uses a pressure-based inflation system automated with a timer. The repetitive ischemia protocol is able to stimulate collateral growth, as shown in a recent publication14. This mouse model of CCG will provide new insight into the process of CCG at cellular and molecular levels and can be used to validate potential targets to promote CCG.
研究方案
The described animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Northeast Ohio Medical University.
1. Surgical preparation
NOTE: For the RI protocol, use C57BL/6 mice of either sex weighing at least 25 g. Use aseptic technique throughout the surgery.
- Setup
- Sterilize all tools in an autoclave or bead sterilizer. Sterilize delicate materials and implants, such as occluder, tether, and PE tubing, with ethylene oxide (EtO).
- Clean the surgical area by wiping all areas with 70% ethanol. Prepare the area by laying out all tools and supplies on sterile drapes. See Table of Materials for a complete list of supplies.
- Mouse intubation
- Briefly anesthetize the mouse with 3% isoflurane with oxygen (1 L/min flow rate) until the righting reflex is lost. Shave the chest area, the center of the back, and behind the right ear; completely remove loose hair. Inject glycopyrrolate intramuscularly at 0.01-0.02 mg/kg.
- Anesthetize again with 3%-4% isoflurane for 5 min. Place the mouse supine on an incline, restrained from its upper incisors, and use blunted forceps to move the tongue aside. Quickly intubate the mouse with a 20G angiocath using a fiber optic light and a magnifying laryngoscope.
- Place the mouse supine on a warming surgical pad and connect the intubation tube to a small animal ventilator with 3% isoflurane. Confirm intubation by checking for rhythmic bilateral chest rise. Confirm adequate depth of anesthesia by lack of toe pinch response.
- Preparation of the surgical field
- Apply ophthalmic ointment to the eyes to prevent drying.
- Clean the shaved areas with betadine and then 70% ethanol, wiping once in a unidirectional manner.
- Apply electrode cream to the electrocardiogram (EKG) contacts on the surgical pad and tape the mouse's limbs to them. Drape sterile gauze over the lower half of the mouse.
- Monitor breathing rate, temperature, and depth of anesthesia over the course of the surgery using the surgical pad's software interface.
- Left thoracotomy and occluder implantation
- Decrease isoflurane to 2%. Make a 2-3 cm midline incision in the skin on the chest using scissors, then use curved forceps to gently separate the skin and muscle layers on the left side of the chest (Figure 1A).
- Using blunted forceps, gently make an opening through the thoracic wall to expose the heart, typically between the 3rd and 5th intercostals.
- Use a retractor to gently spread the ribs and visualize the heart. Using blunted forceps, tear the pericardium to expose the heart. Locate the left auricle (Figure 1B) and visualize the basal part of the left anterior descending artery (LAD), if possible.
- Pass an 8-0 polypropylene suture through the myocardium underneath the LAD.
NOTE: The distance between the entrance and exit should be about equal to the width of the occluder and perpendicular to the LAD. - Remove retractors. Using pointed forceps, exteriorize the end of the occluder tubing through the chest wall in the second inferior intercostal space and pull the occluder tubing out through the chest wall until the occluder is located over the heart in the chest cavity.
- Tie the occluder securely to the heart using a surgeon's knot (Figure 1C) so that the occluder lies on the heart but does not press into it.
- Briefly inflate the occluder (~10 s) at 10 psi using the push-button inflation device (Figure 2). Check the EKG to confirm ST elevation during inflation and, if possible, visually confirm blanching of the heart apex. Adjust the position or tension of the suture as necessary to confirm ischemia.
- Closing
- Close the flanking ribs with 6-0 polypropylene suture, inserting the chest tube. Evacuate air and blood from the chest, then remove the chest tube. Apply 2% lidocaine to the closed incision in the chest wall.
- Using pointed forceps, tunnel the occluder tubing under the skin of the right shoulder to exteriorize the tubing through the skin behind the right ear. Replace chest muscles and close the skin with a 6-0 polyglactin suture (Figure 1D).
- Flip the mouse to the prone position. Administer ketoprofen subcutaneously at a dosage of 3 mg/kg (using a 1 mg/mL dilution in 0.9% sterile saline) at least 30 min before the end of surgery.
- Tether implantation
- Using small scissors, make a small (1 cm) incision on the skin at the center of the back and gently separate the skin from the subcutaneous fat and muscle tissue.
- Tunnel the occluder tubing to the incision at the center of the back, exteriorize it, and thread it through the tether. Use a 6-0 suture to close the small hole near the ear.
- Use 6-0 polypropylene sutures to fix the tether to the back muscles (Figure 1E). Close the skin over the tether button with 6-0 sutures.
- Recovery
- Remove the intubation tube once the mouse can breathe independently; place the mouse in a post-surgical RI cage (for a single animal; Figure 3) once the foot pinch response has returned. Monitor the mouse continuously until it has regained sufficient consciousness to maintain sternal recumbency. Place the cage on a heat pad until the mouse regains full mobility.
- The next day, administer a second dose of ketoprofen subcutaneously at 3 mg/kg.
- Allow the mouse to rest for 5-7 days before beginning the RI protocol. Monitor mice daily for integrity of instrumentation and change the cages as needed. Provide appropriate enrichment as needed, as mice are single housed for the duration of the RI protocol.
2. Repetitive ischemia
- Check the placement of the occluder by echocardiography on Day 0 of the RI protocol, as previously described15. Observe the decrease in cardiac function during inflation of the occluder.
- Connect the mouse's occluder tubing to the RI inflation system (Figure 4). The system will inflate the occluder to 10 psi for 6 min, 4x a day, with a 3 h break between each inflation (Figure 5).
- After 17 days of RI, re-check cardiac function as in step 2.1.
3. Polymer perfusion and tissue harvest
- At the time of sacrifice, anesthetize the mouse with 3%-4% isoflurane and inject heparin (500 U/kg) intraperitoneally. Use a nose cone to continue isoflurane administration at 2% for at least 5 min, then confirm the adequate depth of anesthesia by lack of toe pinch response.
- Open the chest cavity to expose the heart and thoracic aorta. Cannulate the descending thoracic aorta with PE20 tubing and bisect the inferior vena cava (IVC) to allow outflow; perfuse the heart retrogradely with 1x PBS until the fluid exiting the IVC is clear, followed by 3 mL of 1% lidocaine, then 3 mL of 4% paraformaldehyde (PFA) in PBS. Make a permanent ligation of the LAD at the exact position of the occluder.
- Retrogradely perfuse the heart with a radiopaque reagent until the arterial circuit is filled. Clamp the PE20 tubing with hemostats and allow the polymer to cure for 90 min. Image the heart under a dissection scope.
结果
Out of 136 C57BL/6 mice, including both males and females, the survival rate of the RI surgery was 93.4%, with 80.9% of mice surviving through the entire 17-day RI protocol.
The mouse RI protocol was optimized based on previous animal RI models12,16, which have short episodes of ischemia without permanent injury to the myocardium. During the surgery, functional assessment of the occluder can be done by observing visible blanching of the LV apex and an ST elevation on the EKG during inflation of the occluder, which both return to normal after deflation (Figure 6). After the post-surgical recovery period, the function of the occluder was again checked on Day 0 by echocardiography both before and during the inflation of the occluder. Both ejection fraction (EF) and fractional shortening (FS) are decreased during inflation, which indicates that the occluder was placed correctly and is causing ischemia and reduced function while inflated (Figure 7A). These parameters can be assessed again on Day 17; if the cardiac function during occluder inflation has improved over the 17 days, as seen in Figure 7A, it suggests the ischemia induced by inflation of the occluder was ameliorated by collaterals grown over the RI period, which are now resupplying the collateral-dependent zone (CZ).
Besides the functional assay by echocardiography, another assessment of CCG is to visualize the presence of collateral vessels by perfusion of a radiopaque polymer. Mice are reported to have no native collaterals13, so in a native mouse heart (no surgery or RI), ligation of the LAD followed by retrograde perfusion of the polymer produces a non-filled CZ region downstream of the ligation, where arteries are not filled with polymer because flow has been stopped (Figure 7B). In contrast, the presence of filled arteries in the CZ indicates the presence of collateral circulation. In mice that have undergone the RI protocol, after ligation at the occluder position, the CZ is perfused with polymer via the collaterals developed during the RI period (Figure 7C).
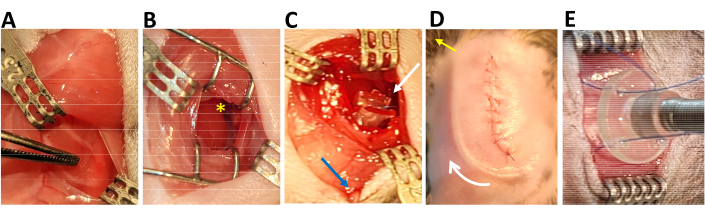
Figure 1: Surgical view of key steps. (A) Location for the thoracotomy indicated by forceps tips after midline incision and retraction of skin and muscle. (B) The heart after thoracotomy; yellow asterisk indicates the location of the left auricle. (C) The placement of the occluder on the heart (white arrow) and the exteriorized tubing through the second inferior intercostal space (blue arrow). (D) The view after the chest is closed; the white arrow shows subcutaneous occluder tubing, and the yellow arrow shows where tubing is exteriorized on the right shoulder. (E) The fixation of the tether to the back. Please click here to view a larger version of this figure.
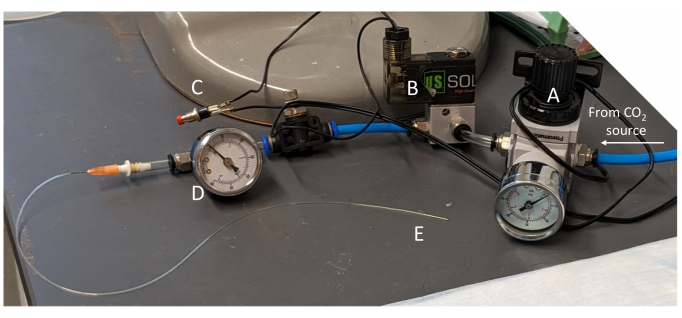
Figure 2: Push-button device for inflating the occluder. The inflation device consists of (A) a regulator, (B) an electric solenoid valve, (C) an on/off push button, and (D) a pressure gauge; it tapers at the end (E) to connect to the occluder tubing. Please click here to view a larger version of this figure.
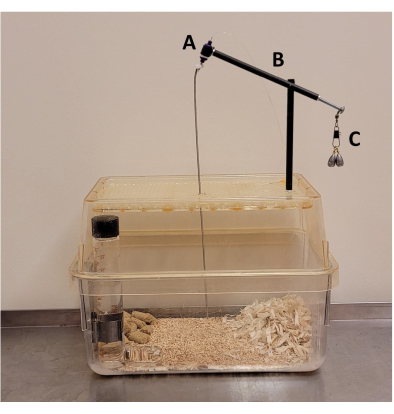
Figure 3: Post-surgical mouse cage. Components include (A) a plastic tether swivel, (B) carbon fiber tubing, and (C) a counterbalance made of snap swivel and sinkers. Please click here to view a larger version of this figure.

Figure 4: Automated inflation system. It consists of (A) a normally closed electric solenoid valve, (B) a pressure gauge, (C) a normally open electric solenoid valve, and (D) an electric panel plugged into a digital programmable timer. When the timer turns the system on, the solenoids switch, and CO2 flows into the system to inflate the occluder. Please click here to view a larger version of this figure.

Figure 5: Timeline of the RI protocol. After RI surgery, mice rest for 5-7 days before initial echocardiography and the start of the RI protocol, which then continues for 17 days until final echocardiography and sacrifice. The RI protocol consists of 4 daily inflations (6 min at 10 psi each) with a 3 h rest between inflations. Please click here to view a larger version of this figure.

Figure 6: Representative electrocardiograms before, during, and after inflation of the occluder. (A) EKG before inflation (the red arrow indicates the start of inflation). (B) EKG during inflation, showing ST elevation. (C) EKG, after deflation, returned to normal. Please click here to view a larger version of this figure.
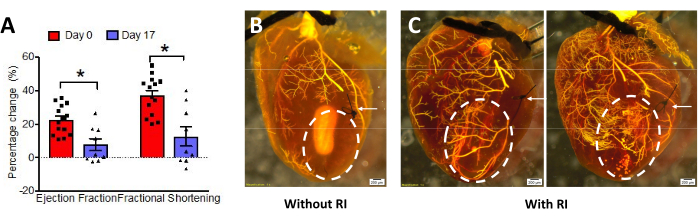
Figure 7: Evaluation of coronary collateral growth (CCG). (A) In wild-type mice, ejection fraction (EF) and fractional shortening (FS) decreased during occluder inflation at Day 0, indicating correct occluder placement. On Day 17, the change in EF% and FS% was significantly less than at Day 0, indicating CCG (n=14 at day 0, n=9 at day 17, unpaired Mann-Whitney U test was used for statistical significance analysis, *p < 0.05. This figure has been modified from14. (B, C) Radiopaque polymer perfusion with and without RI. White arrows show the LAD ligation point. The white dashed circle indicates the collateral-dependent zone (CZ); in non-RI hearts, there is no filling, but in RI hearts, the vessels of the CZ are filled via collaterals. Please click here to view a larger version of this figure.
讨论
Coronary collaterals are a natural bypass for IHD patients. After the failed clinical trials targeting angiogenesis17, promoting coronary collateral development might be a better therapeutic approach for these patients. Different from capillaries derived from angiogenesis, which have only a single layer of endothelial cells, collaterals are mature arteries with the coverage of smooth muscle cells. Collaterals resupply blood flow to the regions of myocardial ischemia caused by obstructive arteries. Understanding the regulation of CCG is vital for developing new targets to treat IHD.
Large animal models using repetitive ischemia to study CCG have been beneficial10,11,12, especially when automated to reduce variance and improve time efficiency18 but cannot fully take advantage of current genetic technologies. Scaling such a CCG model down to the size of a mouse has its challenges, but the benefits are numerous, both practical and scientific: mice cost less to house and require less space while providing a greater toolbox of genetic models.
While several mouse models of CCG have been published in recent years13,16,19,20,21, this model has several distinctions14. Repetitive ischemia (RI) induces collateral growth without loss of or injury to the myocardium caused by myocardial infarction. After occluder implantation, the RI process can be regulated and the program automated, which is beneficial for time efficiency as well as reproducibility18; the automated inflation system is low-cost and easy to program and operate without requiring any computer system. Collateral growth can be evaluated by measurement of cardiac function with and without inflation of the occluder, a good proxy indicator of the existence of coronary collateral flow. Moreover, perfusion with a radiopaque polymer to visualize the vasculature of the heart is simple and low-cost. Though the overall procedure is complex and requires a skilled surgeon, the mouse model of CCG allows for the induction of collateral growth in an adult heart, opening a new area for studying the regulation of CCG and providing a tool to test the therapeutic targets for promoting collateral growth in IHD.
While the data presented here are for wild-type mice at 4-6 months of age, the protocol could be adapted for the different needs of various studies, exploring questions of factors that affect CCG, such as gene regulation, sex differences, aging, and cardiovascular pathologies. We recommend using mice of at least 25 g due to the size of the occluder in relation to the mouse heart. We are also aware that aging is a risk factor for all cardiovascular diseases, and its impact on CCG is unknown. We would expect that the surgical survival rates or the outcome of collateral growth in aged mice might be different from the wild type because of the length and complexity of the surgery or the systemic aging phenotype.
The surgical preparation for the RI protocol is indeed longer and more complicated than a typical MI surgery, involving two implants and the repositioning of the mouse mid-surgery. Attention to several key steps will help to facilitate the process. The most critical step of the procedure is the occluder implantation, which relies on accurately circumscribing the LAD when passing the suture through the myocardium, as well as achieving the correct tension of the suture when tying on the occluder. Accurate positioning of the occluder is straightforward to assess: a successful placement will result in apical blanching as well as ST elevation when, and only when, the occluder is inflated; otherwise, the myocardium and EKG appear normal. It is important to check the EKG with and without inflation during the RI surgery as well as the cardiac function with and without inflation after surgery on Day 0. If a mouse has a myocardial infarction without inflation of the occluder, the animal should be removed from the study. Before occluders are implanted, they need to be tested to optimal pressure. Tether implantation is another key step because the tether protects the occluder tubing from damage by the mouse, which would prematurely terminate the RI protocol. Improper placement can cause skin necrosis.
In summary, this mouse model of CCG via repetitive ischemia is a valuable tool for the study of the mechanism and regulation of CCG and for screening therapeutics developed for the treatment of IHD.
披露声明
The authors have nothing to disclose.
致谢
The authors thank Weiguo Wan, Cody Juguilon, Iyanuoluwa Ogunmiluyi, and Devan Richardson for their contributions to the methods discussed here. This work was supported by 1R15HL115540-01 and 1 R01 HL137008-01A1.
材料
| Name | Company | Catalog Number | Comments |
| #5/45 degree forceps | Fine Science Tools | 11251-35 | |
| 1/4" Closed Brass Electric Solenoid Valve | U.S. Solid | USS2-00054 | inflation system |
| 1/4" Open Brass Electric Solenoid Valve | AceCrew | inflation system | |
| 1/4" pneumatic tubing | China SNS Pneumatic Co.,Ltd | APU1/4-32.8ft | push-button device |
| 1/4" push-in connectors | RuoFeng | 543Y | push-button device |
| 1/8" brass fittings | Edge Industrial | inflation system | |
| 2 Position Pneumatic Electric Solenoid Valve | U.S. Solid | USS- PSV00033 | push-button device |
| 20G angiocath | BD | 381703 | |
| 45 degree Castroviejo needle holders | Roboz | RS-6421 | |
| 6-0 polyglactin sutures | DemeTECH | G176011B13M | |
| 6-0 polypropylene sutures | AD Sugical | XS-P618R11 | |
| 70% Ethanol | |||
| 8-0 polypropylene sutures | DemeTECH | PM19800, 65G0P | |
| Betadine | Purdue Products | 367618150085 | |
| Blunt nosed scissors | World Precision Intruments | 500366 | |
| Carbon fiber arrow shaft | post-surgical cage; cut to 12.5 cm | ||
| Cotton swabs (3") | Puritan | 872-PC DBL | |
| Curity Gauze Sponges (2x2) | Cardinal Health | 2146 | |
| Dipsey swivel sinkers | Water Gremlin | post-surgical cage | |
| Electrode cream | Signacreme | 17-05 | |
| Glycopyrrolate | Westward | 0143-9679-01 | |
| Hartman hemostats | Fine Science Tools | 13003-10 | |
| Isoflurane | Covetrus | 29404 | |
| Ketofen (ketoprofen) | zoetis | 10004031 | |
| Lidocaine (2%) | Covetrus | 14583 | |
| MICROFIL (yellow) | Flow Tek | MV-122 | |
| Mini Push Button | Interactivia | E-SWC-PBM-PBS-105 | push-button device |
| Miniature Air Pressure Regulator | PneumaticPlus | PPR2-N02BG-4 | push-button device |
| Mini-Colibri spring retractor | Fine Science Tools | 17000-01 | |
| MiniVent ventilator | Harvard Apparatus | 73-0044 | |
| Occluder | Custom made | ||
| Octagon handled forceps | Fine Science Tools | 11041-08 | |
| Ohan Rodent Intubation System | BMR Supply | Ohan-201 | |
| Paraformaldehyde solution 4% in PBS | Santa Cruz | sc-281692 | |
| PE20 tubing | |||
| PE50 tubing | |||
| Plastic swivel (1 channel) | Instech | 375/25PS | post-surgical cage |
| Premixed PBS Buffer, 10x | Roche | 11666789001 | Diluted to 1x |
| Pressure Gauge | PIC Gauges | 102D-158D-10/32 | push-button device |
| Programmable Digital Outlet Timer | BN-LINK | BND-60/SU105 | inflation system |
| Puralube Vet Opthalmic Ointment | Dechra | 17033-211-38 | |
| Retractors w/ 18200-07 elastomer | Fine Science Tools | 18200-10 and 18200-11 | |
| Rodent Surgical Monitor+ | Scintica | 900-0053-01 | |
| Round handled suture tying forceps | Fine Science Tools | 18026-10 | |
| Snap-lock barrel swivel (size 5) | Eagle Claw | 01032-005 | post-surgical cage |
| Straight needle holders | Fine Science Tools | 12060-01 | |
| Tether | Instech | PS62 |
参考文献
- Tsao, C. W., et al. Heart disease and stroke statistics-2023 update: A report from the American heart association. Circulation. 147 (8), e93-e621 (2023).
- Chilian, W. M., et al. Coronary collateral growth--back to the future. J Mol Cell Cardiol. 52 (4), 905-911 (2012).
- Liu, Z., et al. Coronary collateralization shows sex and racial-ethnic differences in obstructive artery disease patients. PLoS One. 12 (10), e0183836 (2017).
- Kinnaird, T., Stabile, E., Zbinden, S., Burnett, M. S., Epstein, S. E. Cardiovascular risk factors impair native collateral development and may impair efficacy of therapeutic interventions. Cardiovasc Res. 78 (2), 257-264 (2008).
- Traupe, T., Gloekler, S., de Marchi, S. F., Werner, G. S., Seiler, C. Assessment of the human coronary collateral circulation. Circulation. 122 (12), 1210-1220 (2010).
- Schaper, W. Collateral vessels reduce mortality. Eur Heart J. 33 (5), 564-566 (2012).
- Meier, P., et al. The impact of the coronary collateral circulation on mortality: a meta-analysis. Eur Heart J. 33 (5), 614-621 (2012).
- Hirode, G., Wong, R. J. Trends in the prevalence of metabolic syndrome in the United States, 2011-2016. JAMA. 323 (24), 2526-2528 (2020).
- Turhan, H., et al. Impaired coronary collateral vessel development in patients with metabolic syndrome. Coron Artery Dis. 16 (5), 281-285 (2005).
- Ishikawa, K., et al. Development of a preclinical model of ischemic cardiomyopathy in swine. Am J Physiol Heart Circ Physiol. 301 (2), H530-H537 (2011).
- Rys, R., et al. An automated coronary artery occlusion device for stimulating collateral development in vivo. J Pharmacol Toxicol Methods. 48 (2), 111-118 (2002).
- Toyota, E., et al. Vascular endothelial growth factor is required for coronary collateral growth in the rat. Circulation. 112 (14), 2108-2113 (2005).
- Zhang, H., Faber, J. E. De novo collateral formation following acute myocardial infarction: Dependence on CCR2⁺ bone marrow cells. J Mol Cell Cardiol. 87, 4-16 (2015).
- Jamaiyar, A., et al. The essential role for endothelial cell sprouting in coronary collateral growth. J Mol Cell Cardiol. 165, 158-171 (2022).
- Yin, L., et al. Induction of vascular progenitor cells from endothelial cells stimulates coronary collateral growth. Circ Res. 110 (2), 241-252 (2012).
- Lavine, K. J., Kovacs, A., Weinheimer, C., Mann, D. L. Repetitive myocardial ischemia promotes coronary growth in the adult mammalian heart. J Am Heart Assoc. 2 (5), e000343 (2013).
- Simons, M., et al. Clinical trials in coronary angiogenesis: issues, problems, consensus: An expert panel summary. Circulation. 102 (11), E73-E86 (2000).
- Leavesley, S. J., Ledkins, W., Rocic, P. A device for performing automated balloon catheter inflation ischemia studies. PLoS One. 9 (4), e95823 (2014).
- Aghajanian, A., et al. Decreased inspired oxygen stimulates de novo formation of coronary collaterals in adult heart. J Mol Cell Cardiol. 150, 1-11 (2021).
- He, L., et al. Genetic lineage tracing discloses arteriogenesis as the main mechanism for collateral growth in the mouse heart. Cardiovasc Res. 109 (3), 419-430 (2016).
- Das, S., et al. A unique collateral artery development program promotes neonatal heart regeneration. Cell. 176 (5), 1128-1142.e18 (2019).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。