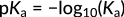An acid protonates a water molecule to form a hydronium ion and its conjugate base. In the reverse reaction, the conjugate base accepts a proton from the hydronium ion. The extent to which an acid dissociates in water defines its strength.
In effect, strong acids completely dissociate in water, and the equilibrium lies on the side of the products, meaning that their solutions contain a high concentration of hydronium ions.
In comparison, weak acids only partially dissociate in water, so the equilibrium favors the reactants. Therefore, their solutions mostly contain undissociated acid molecules with only a few hydronium ions.
The degree of dissociation of a weak acid can be measured using the equilibrium constant, Keq.
In a dilute acid solution, because the change in the concentration of water is negligible, its value essentially remains constant.
Thus, a new equilibrium constant — called the acidity constant, Ka— is defined. Note that the expression has the concentration of hydronium ions in the numerator.
A higher Ka corresponds to a larger hydronium ion concentration, thereby indicating a stronger acid.
The values of Ka range several orders of magnitude for various organic acids. Hence, pKa, expressed as the negative logarithm of Ka, is normally used to indicate the strengths of different acids.
The minus sign in the expression implies that the higher the pKa, the smaller the Ka, and hence the weaker the acid. Therefore, benzoic acid with a pKa of 4.2 is weaker than hydrobromic acid with a pKa of −9.
Additionally, a pKa value can also be used to determine the strength of a base, since every base has a conjugate acid associated with it. The stronger the conjugate acid, the weaker its base.
For example, the pKa of the conjugate acid of methanol is −2.5, which is less than the pKa of the conjugate acid of methylamine.
Consequently, the conjugate acid of methanol is stronger than the conjugate acid of methylamine, and methanol is a weaker base than methylamine.