Method Article
Comprehensive Analysis of Procoagulant Platelets Exhibiting Features of Necrosis, Apoptosis and Platelet Activation
In This Article
Summary
The present protocol provides a comprehensive set of procedures necessary to analyze procoagulant platelets, which exhibit overlapping features of necrosis, apoptosis, and platelet activation.
Abstract
Platelets circulating in the bloodstream are relatively quiescent but become "activated" upon encountering stimulants or "agonists" at the site of blood vessel injury. Proaggregatory and procoagulant platelets represent two distinct populations of activated platelets. While proaggregatory platelets facilitate the cessation of bleeding, or "hemostasis," by forming a plug of platelets clumped together through fibrinogen bridges, procoagulant platelets dramatically accelerate the coagulation cascade, culminating in fibrin clot formation. An interesting aspect of procoagulant platelets is that their morphology exhibits certain features of "necrosis" and "apoptosis." They may thus represent a form of cell death in platelets, albeit with an important role in thrombosis and hemostasis. This article introduces the concept of procoagulant platelets, their relevance to health and disease, and a comparison of existing methods for their analysis. It then provides comprehensive protocols for analyzing procoagulant platelets, investigating the mechanisms of their formation, and assessing their prothrombotic role in facilitating coagulation. The article concludes with a discussion of key steps, limitations, and troubleshooting principles for the described methods.
Introduction
There are at least two distinct populations of activated platelets1,2. Pro-aggregatory platelets are characterized by high integrin activation and low, if any, PS exposure. On the other hand, procoagulant platelets are characterized by low integrin activity and high PS exposure1,2, providing a surface for the assembly of tenase and prothrombinase complexes3, which is 105-106-fold and 300000-fold more active than individual soluble phase factor IXa and Xa, respectively4. Procoagulant platelets thus dramatically accelerate coagulation. An interesting aspect of procoagulant platelets is that their morphology resembles certain features of "necrosis" such as microvesiculation, ballooning of the cell with cytoskeletal disruption, and loss of membrane integrity, as well as those of "apoptosis" such as loss of membrane phospholipid asymmetry with exposure of phosphatidylserine in the outer leaflet5,6. In other words, procoagulant platelet formation may represent a form of cell death in platelets, albeit with an important physiological function in hemostasis.
Procoagulant platelets are significantly associated with thrombotic disorders. While most healthy individuals do not have circulating procoagulant platelets, ~30% of normal donor platelets adopt a procoagulant phenotype ex vivo following exposure to strong agonists such as thrombin and collagen7. Circulating procoagulant platelets have been reported in trauma, where their formation may reflect activation by histone H48,9. In most prothrombotic disorders, however, increased levels of procoagulant platelets are only detected following ex vivo stimulation10. For example, patients with acute stroke in whom >51.1% of their platelets were converted to procoagulant platelets (also known as COATed platelets) by collagen and thrombin had a hazard ratio of 10.72 for recurrent stroke within 30 days compared to patients with lesser procoagulant platelet formation11. Similar results have been reported in patients with transient ischemic attacks and carotid atherosclerosis12. In contrast, the bleeding disorder Scott syndrome results from a mutation of ANO6, which encodes the phospholipid scramblase TMEM-16F, leading to deficient platelet PS exposure13. Idiopathic bleeding disorders and intracranial bleeding may be associated with a decreased ability to generate procoagulant platelets14.
Hence, assessing procoagulant platelets is part of any analysis of platelet function not only during basic investigations into mechanisms of platelet activation and consequent thrombosis and hemostasis, but also during clinical analysis for risk of thrombosis or bleeding in patients during various pathological states. An International Society on Thrombosis and Haemostasis (ISTH) panel recommended the use of Annexin V binding and P-selectin expression by flow cytometry for distinguishing procoagulants from other platelet subpopulations15. The article also discusses the various methods that can be used to analyze procoagulant and apoptotic platelets but falls short of describing the processes in detail. These methods include detection of (1) platelet activation by PAC1/JonA or fibrinogen binding (flow cytometry); (2) alpha-granule secretion by P-selectin expression (flow cytometry); (3) PS exposure by Annexin V/lactadherin binding (flow cytometry); (4) loss of membrane integrity by GSAO labeling (flow cytometry); (5) morphological changes like ballooning (microscopy); (6) detection of caspase activation by caspase assay (immunoblotting/luminometry/flow cytometry) or degradation of cytoskeletal substrate gelsolin (immunoblotting); (7) loss of mitochondrial membrane potential by mitochondrial potential-sensitive dyes such as JC-1/Mitotracker (flow cytometry); (8) mitochondrial intrinsic apoptotic markers Bax, Bak and cytochrome c release (immunoblotting); (9) procoagulant function by thrombin generation assay and coagulation factor Xa/Va binding (flow cytometry, microscopy); (10) cytosolic and mitochondrial calcium rise by fluorescent calcium sensitive dyes (flow cytometry, fluorometry, microscopy).
The present study delves into comprehensive protocols for the analysis of procoagulant platelets as well as distinguishing them from proaggregatory and apoptotic platelets. Most procedures described rely on flow cytometry that has the advantages of (1) being readily available and easy to use, (2) requiring low sample volume, and (3) allowing simultaneous detection of multiple subpopulations of platelets (proaggregatory, procoagulant, and apoptotic)15. These flow cytometry-based protocols are supplemented with functional assays of procoagulant activity based on coagulation factor binding and clot-based thrombin generation assays.
Protocol
Human participants were recruited in the study for peripheral venous blood sampling after obtaining written informed consent, strictly following the recommendations and approval of the Institutional Review Board of Cleveland Clinic Lerner Research Institute, with all study methodologies conforming to the standards set by the Declaration of Helsinki. Healthy adult participants above 18 years of age were included, while those younger than 18 years, individuals with a recent history of thrombotic events in the past six months, those with a history of alcoholism or drug abuse, and participants who had used anti-platelet or anti-coagulant medications in the past four weeks were excluded. A detailed description of materials and reagents used in the protocols can be found in the Table of Materials.
1. Platelet preparation
- Draw peripheral venous blood samples into ACD anticoagulant (1:9 v/v) from human participants after obtaining written informed consent, strictly following the recommendations approved by the Institutional Review Board.
- Centrifuge the blood collected in ACD at 100 x g for 20 min at 22 °C to obtain platelet-rich plasma (PRP) as the supernatant.
- Centrifuge the PRP at 800 x g for 7 min at 22 °C after adding 3 µM of PGE1 and 2 mM of EDTA.
- Discard the supernatant and resuspend the platelet pellet in buffer A (see Table of Materials) by gentle pipetting.
- Centrifuge the platelets resuspended in buffer A at 800 x g for 7 min at 22 °C.
- Resuspend the platelet pellet in buffer B (see Table of Materials) by gentle pipetting.
- Adjust the final platelet count to 1 × 107/mL using an automated cell counter.
NOTE: Perform all steps under sterile conditions and take precautions to maintain the platelets in a resting state by avoiding exposure to excessive shear during pipetting.
2. Analysis of procoagulant platelets by flow cytometry
- Supplement washed human platelets with 2.5 mM calcium (0.5 µL of stock CaCl2 0.5 M solution in 100 µL of platelet suspension).
- Keep one fraction of platelets unstimulated and stimulate the remaining fractions with thrombin (0.1 U/mL, 0.25 U/mL, 0.5 U/mL), convulxin (20 ng/mL, 50 ng/mL, 100 ng/mL), or their combination for 15 min at room temperature.
- After stimulation, add 1 µL each of PE-Annexin V, FITC-PAC1, and APC-anti-human CD62P antibody to 100 µL of stimulated platelet suspensions.
- Incubate the platelets in the dark at room temperature for 30 min.
- Fix the platelets by adding an equal volume (1:1) of 2% formalin.
- Analyze the samples by flow cytometry to quantify procoagulant platelets15,16.
- Draw an amorphous gate to encompass platelets, separating them from noise and multi-platelet particles.
- Collect all fluorescence data using four-quadrant logarithmic amplification for 10,000 events in the platelet gate from each sample.
- Define regions for platelets based on fluorescence positivity or negativity for phosphatidylserine (PS) exposure (Annexin V binding), P-selectin (CD62P) expression, and integrin αIIbβ3 activation (PAC1 binding).
- Consider platelets positive for both PS exposure (Annexin V binding) and P-selectin (CD62P) expression but negative for integrin αIIbβ3 activation (PAC1 binding) as procoagulant platelets (Figure 1) (Table 1).
- Similarly, consider platelets positive for both integrin αIIbβ3 activation (PAC1 binding) and P-selectin (CD62P) expression but negative for PS exposure (Annexin V binding) as proaggregatory platelets.
NOTE: Platelets negative for both integrin αIIbβ3 activation (PAC1 binding) and P-selectin (CD62P) expression but positive for PS exposure (Annexin V binding) are likely apoptotic platelets15,16.
3. Analysis of mitochondrial calcium by flow cytometry
- Dilute washed human platelets to 1 × 106/mL and supplement with 2.5 mM calcium (0.5 µL of stock CaCl2 0.5 M solution in 100 µL platelet suspension).
- Label the platelets with 5 µM Rhod-2 AM (for mitochondrial calcium) for 30 min in the dark.
- Gate platelets appropriately, as described in step 2.
- Analyze events in the platelet gate for time-dependent changes in the mean fluorescence of acquired events over 5 min using a density scatter plot of PE fluorescence (for Rhod-2 AM) versus time.
- Record baseline calcium levels for the first 1 min.
- Add thrombin (0.1 U/mL, 0.25 U/mL, or 0.5 U/mL), convulxin (20 ng/mL, 50 ng/mL, or 100 ng/mL), or their combination, and continue acquiring data for another 4 min17.
4. Analysis of mitochondrial membrane potential by flow cytometry
- Supplement washed human platelets with 2.5 mM calcium (0.5 µL of stock CaCl2 0.5 M solution in 100 µL of platelet suspension).
- Keep one fraction of platelets unstimulated and stimulate the remaining fractions with thrombin (0.1 U/mL, 0.25 U/mL, or 0.5 U/mL), convulxin (20 ng/mL, 50 ng/mL, or 100 ng/mL), or their combination for 15 min at room temperature.
- After stimulation, add mitochondria labeling dye (see Table of Materials) at a final concentration of 500 nM to the stimulated platelet suspensions.
- Incubate the platelets for 30 min in the dark.
- Fix the platelets by adding an equal volume of 2% formalin.
- Analyze samples by flow cytometry17.
- Gate platelets appropriately as described in step 2.
- Analyze events in the platelet gate for a drop in fluorescence in the PE channel (for the mitochondria labelling dye) of the flow cytometer.
5. Analysis of caspase 3 and caspase 8 activity by flow cytometry
- Supplement washed human platelets with 2.5 mM calcium (0.5 µL of stock CaCl2 0.5 M solution in 100 µL of platelet suspension).
- Keep one fraction of platelets unstimulated and stimulate the remaining fractions with thrombin (0.1 U/mL, 0.25 U/mL, or 0.5 U/mL), convulxin (20 ng/mL, 50 ng/mL, or 100 ng/mL), or their combination for 15 min at room temperature.
- After stimulation, add either 1:1000 (v/v) apoptosis detection reagent (see Table of Materials) (for both intrinsic and extrinsic apoptosis pathways) or 1:300 (v/v) FITC-IETD-FMK (for caspase 8 of the extrinsic apoptosis pathway) to the stimulated platelet suspensions.
- Incubate the platelets for 30 min in the dark.
- Fix the platelets by adding an equal volume of 2% formalin.
- Analyze samples by flow cytometry17.
- Gate platelets appropriately, as described in step 2.
- Analyze events in the platelet gate for fluorescence in the FITC channel (for either caspase 3/7 or caspase 8) of the flow cytometer.
6. Analysis of prothrombin binding by flow cytometry
- Conjugate bovine prothrombin with Alexa Fluor 488 dye using a protein labeling kit (see Table of Materials), following the manufacturer's instructions.
- Supplement washed human platelets with 2.5 mM calcium (0.5 µL of stock CaCl2 0.5 M solution in 100 µL platelet suspension).
- Keep one fraction of platelets unstimulated and stimulate the remaining fractions with thrombin (0.1 U/mL, 0.25 U/mL, or 0.5 U/mL), convulxin (20 ng/mL, 50 ng/mL, or 100 ng/mL), or their combination for 15 min at room temperature.
- After stimulation, add AF488-conjugated bovine prothrombin (100 µg/mL) and 1 µL each of PE-Annexin V and APC anti-human CD62P antibody to 100 µL of stimulated platelet suspensions.
- Fix the platelets by adding an equal volume of 2% formalin.
- Analyze samples by flow cytometry17 for the quantification of prothrombin binding to platelets.
- Draw an amorphous gate to encompass platelets separate from noise and multi-platelet particles.
- Collect all fluorescence data using four-quadrant logarithmic amplification for 10,000 events in the platelet gate from each sample.
- Draw regions for platelets with fluorescence positive or negative for PS exposure (Annexin V binding), P-selectin (CD62P) expression, and AF488-prothrombin (prothrombin binding).
- Determine the proportion of all platelet events, as well as the proportion of platelets positive for both PS exposure (Annexin V binding) and P-selectin (CD62P) expression (procoagulant platelets), that are positive for prothrombin binding.
7. Analysis of prothrombin binding by confocal microscopy
- Conjugate bovine prothrombin with Alexa Fluor 488 dye using a protein labeling kit (see Table of Materials), following the manufacturer's instructions.
- Supplement washed human platelets with 2.5 mM calcium (0.5 µL of stock CaCl2 0.5 M solution in 100 µL platelet suspension).
- Keep one fraction of platelets unstimulated and stimulate the remaining fractions with thrombin (0.1 U/mL, 0.25 U/mL, or 0.5 U/mL), convulxin (20 ng/mL, 50 ng/mL, or 100 ng/mL), or their combination for 15 min at room temperature.
- After stimulation, add AF488-conjugated bovine prothrombin (100 µg/mL) and 1 µL each of PE-Annexin V and APC anti-human CD62P antibody to 100 µL of stimulated platelet suspensions.
- Fix the platelets by adding an equal volume of 2% formalin.
- Pellet the fixed platelets onto poly-D-lysine-coated coverslips.
- Mount coverslips on microscopy slides using an anti-fade solution.
- Alternatively, coat coverslips with collagen (100 µg/mL) for 1 h in a humid chamber.
- Block the coated coverslips with 0.5% BSA in PBS for 1 h.
- Label the platelets with AF488-conjugated bovine prothrombin (100 µg/mL) and 1 µL each of PE-Annexin V and APC anti-human CD62P antibody.
- Allow labeled platelets to adhere for 20 min on collagen-coated coverslips.
- Wash coverslips with adherent platelets three times with PBS.
- Fix coverslips with 2% paraformaldehyde for 1 h.
- Mount coverslips on microscopy slides using an anti-fade solution.
- Observe the slides under a confocal microscope at 63× objective magnification.
- Analyze the images using Fiji software for fluorescence quantification.
8. Clot-based platelet phospholipid-dependent thrombin generation assay
- Supplement washed human platelets with 2.5 mM calcium (0.5 µL of stock CaCl2 0.5 M solution in 100 µL of platelet suspension).
- Keep one fraction of platelets unstimulated and stimulate the remaining fractions with thrombin (0.1 U/mL, 0.25 U/mL, or 0.5 U/mL), convulxin (20 ng/mL, 50 ng/mL, or 100 ng/mL), or their combination for 15 min at room temperature.
- Add 100 µL of treated platelets to a mixture of 50 µL of pooled normal plasma and 50 µL of low-turbidity kaolin solution (20 mg/mL), preincubated at 37 °C for 5 min.
- Add 5 mM of CaCl2 to the mixture.
- Monitor the clot formation by turbidimetry on a microplate reader, measuring absorbance at 660 nm every 60 s for 1 h.
Results
A proportion of activated platelets turn "procoagulant" with a characteristic increase in surface expression of both phosphatidylserine (PS) and P-selectin, which distinguishes them from "apoptotic" platelets that are positive only for PS exposure as well as "proaggregatory" platelets that are positive for P-selectin expression. We found that thrombin induces a dose-dependent increase in the proportion of procoagulant platelets positive for both P-selectin and PS expression as detected by binding of FITC-anti-CD62P antibody and PE-Annexin V, respectively (Figure 2). The generation of procoagulant platelets is dependent upon mitochondrial calcium influx18,19 along the electrochemical gradient across the inner mitochondrial membrane upon agonist-induced cytosolic calcium elevations as well as the consequent cyclophilin D (CypD)-dependent mitochondrial permeability transition pore (mPTP) formation20. In consistence, thrombin-induced increase in procoagulant activity was associated with an increase in mitochondrial calcium (Figure 3) and a drop in mitochondrial membrane potential (Figure 4) as measured using Rhod-2 and Mitotracker Red dyes, respectively. Stimulated platelets exhibit features of apoptosis, including Bax/Bak accumulation21,22; whether this activates caspase23,24 and contributes to PS exposure25 remains controversial. Thrombin stimulation of platelets was associated with activation of caspase 8, but not terminal caspases 3 and 7 (Figure 5), as analyzed by FITC-IETD-FMK and apoptosis detection reagent, respectively.
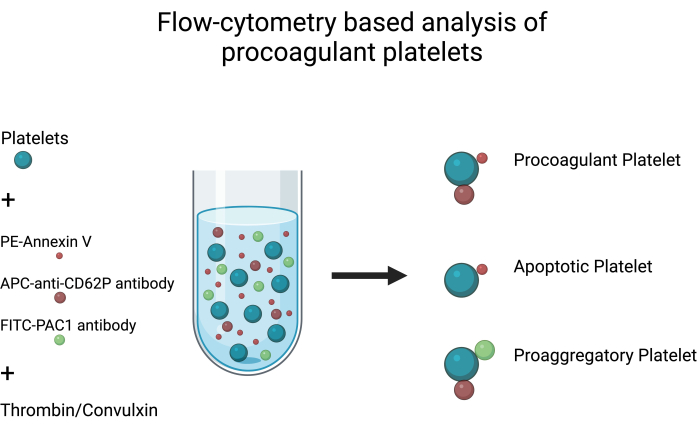
Figure 1: Analysis of procoagulant platelets. Schematic diagram depicting analysis of procoagulant platelets by flow cytometry using FITC-PAC1, APC-anti-CD62P antibodies, and PE-Annexin V as fluorescent probes to detect integrin activation, P-selectin expression, and PS exposure, respectively. Please click here to view a larger version of this figure.

Figure 2: P-selectin expression and phosphatidylserine exposure in platelets upon thrombin stimulation. Representative dot plots showing platelets positive for P-selectin expression and/or PS exposure in (A) unstimulated platelets and upon exposure to thrombin at doses of (B) 0.1 U/mL, (C) 0.25 U/mL and (D) 0.5 U/mL as detected by FITC-anti-CD62P and PE-Annexin V fluorescence using flow cytometry. Figures in parentheses indicate the proportion of total platelets in each quadrant of the plot. Please click here to view a larger version of this figure.

Figure 3: Time-dependent changes in mitochondrial calcium levels in platelets upon thrombin exposure. Representative density plots showing time-dependent changes in fluorescence due to Rhod-2 dye indicating mitochondrial calcium levels in platelets upon exposure to thrombin at doses of (A) 0.1 U/mL, (B) 0.25 U/mL and (C) 0.5 U/mL as detected by flow cytometry. Please click here to view a larger version of this figure.

Figure 4: Mitochondrial membrane potential in platelets upon thrombin stimulation. This figure shows representative histogram overlays depicting fluorescence due to mitochondria labeling dye, indicating mitochondrial membrane potential in platelets upon exposure to different doses of thrombin, as detected by flow cytometry. Please click here to view a larger version of this figure.

Figure 5: Caspase 3/7 and Caspase 8 activities in platelets upon thrombin stimulation. Representative histogram overlays showing fluorescence due to (A) apoptosis detection reagent and (B) FITC-IETD-FMK, respectively, indicating caspase 3/7 and caspase 8 activities in platelets upon exposure to different doses of thrombin as detected by flow cytometry. Please click here to view a larger version of this figure.
| Marker | Procoagulant Platelets | Proaggregatory platelets | Apoptotic platelets |
| Integrin activation (PAC1 binding) | No | Yes | No |
| Granule secretion | Yes | Yes | No |
| (P-selectin expression) | |||
| PS exposure | Yes | No | Yes |
| (Annexin V binding) |
Table 1: Flow cytometry markers for identifying platelet populations after stimulation. This table shows flow cytometry markers used to detect distinct populations of platelets that emerge after stimulation of platelets with strong agonists such as thrombin and convulxin.
Discussion
Procoagulant platelets demonstrate marked and sustained increases in intracellular calcium upon stimulation26, but may be derived through different mechanisms. They are generated upon strong agonist stimulation with collagen and thrombin through distinct mediators, including most prominently mitochondrial calcium influx18,19 along the electrochemical gradient across the inner mitochondrial membrane upon agonist-induced cytosolic calcium elevations. Once the mitochondrial calcium reaches a certain threshold, it activates mitochondrial permeability transition pore (mPTP) formation in a cyclophilin D (CypD)-dependent manner20. When mitochondria are permeabilized to calcium and can no longer serve as sinks for the cytosolic calcium, supramaximal calcium signaling is observed after mPTP formation26. The sustained high levels of cytosolic calcium, in turn, activate the calcium-dependent phospholipid scramblase TMEM16F activation, leading to PS exposure27,28. PS exposure is accompanied by the inactivation of integrin αIIbβ3 by a dual mechanism involving calpain-dependent cleavage of the integrin β3 cytoplasmic tail as well as TMEM16F-dependent phospholipid scrambling itself29,30. Thus, procoagulant platelets, unlike proaggregatory platelets, have an inactive integrin. In addition, PS exposure provides a surface for the binding of coagulation factors, facilitating the assembly of tenase and prothrombinase complexes3. Procoagulant platelets thus dramatically accelerate coagulation4.
PS exposure and consequent procoagulant activity can also be induced by caspase-dependent cleavage of scramblase XKR8 in platelets undergoing intrinsic apoptosis, independent of activation25,31. Although stimulated platelets exhibit features of intrinsic apoptosis, including Bax/Bak accumulation21,22, whether this activates caspase23,24 and contributes to PS exposure25 remains controversial. Extrinsic apoptosis by TNFα or FasL has not been reported in platelets despite the presence of most components and some evidence for caspase 8 activation32. Platelets upon prolonged stimulation undergo MLKL-mediated necroptosis17, which is known to induce PS exposure independent of CypD or caspase33.
This study provided a method for detecting procoagulant platelets using PS exposure (Annexin V binding), P-selectin (CD62P) expression, and integrin αIIbβ3 activation (PAC1 binding) as markers. These markers can help effectively distinguish procoagulant platelets from apoptotic and proaggregatory platelets15. However, annexin V binding requires the presence of high calcium levels in the medium. Hence, heparin or other thrombin inhibitors must be used when using platelet-rich plasma or whole blood samples. Further, fibrin polymerization inhibitor GPRP is employed when thrombin must be used as one of the agonists with these samples34,35. Lactadherin or GSAO can be used as alternative markers to Annexin V, but they have their own limitations. While lactadherin is not specific to PS and binds integrins36,37, GSAO is not commercially available and can only be sourced through research collaboration38.
This article also provided supplemental methods to investigate mechanisms of PS exposure, including mitochondrial calcium influx and mitochondrial permeability transition pore formation (loss of mitochondrial membrane potential). It should be noted here that these methods can be complemented by cytosolic calcium measurements using Fluo-4 and Fluo-5N dyes. As it remains unclear if caspase activation contributes to PS exposure in stimulated platelets, we also enumerate protocols for caspase 8 and caspase 3/7 activation assays. The true "procoagulant" nature of platelets can only be affirmed by their ability to bind coagulation factors and promote thrombin generation. Thus, a thorough study of platelets for PS exposure (Annexin V binding), P-selectin (CD62P) expression and integrin αIIbβ3 activation (PAC1 binding), mitochondrial calcium influx, mitochondrial membrane potential, caspase activity along with coagulation factor (prothrombin) binding and platelet phospholipid dependent thrombin generation/coagulation as described in the present article would be necessary and sufficient for a comprehensive analysis of procoagulant platelets.
Disclosures
Authors have no competing interests to disclose.
Acknowledgements
Paresh P. Kulkarni and Keith R. McCrae, respectively, acknowledge Fellow and Pilot grant awards funded by VeloSano, Cleveland Clinic Foundation.
Materials
| Name | Company | Catalog Number | Comments |
| Acid Citrate Dextrose (ACD) solution (For 1000 mL) | Tri- Sodium Citrate- 22 g Citric Acid- 8 g Dextrose- 24.5 g Water- Make up volume to 1000 mL | ||
| Alexa Fluor 488 protein labelling kit | Invitrogen | A10235 | |
| APC Mouse Anti-Human CD62P | BD Pharmingen | 550888 | |
| Bovine Prothrombin | Prolytix | BCP-1010 | |
| Buffer A (Platelet Preparation) | M.W Conc. in 1X For 100 mL 10X solution HEPES 238.30 20 mM 4.766 g NaCl 58.44 134 mM 7.83 g KCl 74.55 2.9 mM 216.19 mg MgCl2 203.30 1 mM 203.30 mg NaH2PO4 156.01 0.34 mM 53.04 mg NaHCO3 84 01 12 mM 1.01 g Water to 100 mL after adjusting pH to 6.2 Dilute 10X solution 1:10 (v/v) with Milli Q water just before platelet preparation to obtain the 1X solution that needs to be supplemented with the following Conc. in 1X For 1 ml 1X solution EGTA 1 mM Add 10 μL (1:100 v/v) 100 mM EGTA Glucose 5 mM Add 5 μL (1:200 v/v) 1 M glucose PGE1 3 μM Add 3 μL (1:333 v/v) 1 mM PGE1 solution | ||
| Buffer B (Platelet Preparation) | M.W Conc. in 1X For 100 mL 10X solution HEPES 238.30 20 mM 4.766 g NaCl 58.44 134 mM 7.83 g KCl 74.55 2.9 mM 216.19 mg MgCl2 203.30 1 mM 203.30 mg NaH2PO4 156.01 0.34 mM 53.04 mg NaHCO3 84 01 12 mM 1.01 g Water to 100 mL after adjusting pH to 7.4 Dilute 10X solution 1:10 (v/v) with Milli Q water just before platelet preparation to obtain the 1X solution that needs to be supplemented with the following Conc. in 1X For 1 ml 1X solution Glucose 5 mM Add 5 μL (1:200 v/v) 1 M glucose | ||
| CaCl2 (0.5 M) (For 50ml) | Molecular weight of CaCl2.2H2O = 147.02 Dissolve 3.675 g of CaCl2.2H2O in 50 ml Milli Q water | ||
| CellEvent Caspase-3/7 Detection Reagents Green | Invitrogen | C10423 | Apoptosis detection reagent |
| Convulxin | Enzo Life Sciences | ALX-350-100 | |
| EDTA (0.5 M; pH 8) (For 100 mL) | Molecular Weight of EDTA Na2.2H2 O: 372.24g Weigh 18.612 g and suspend in 50 ml Milli Q water and check the pH (pH~4) Slowly add 10N NaOH with stirring and monitor the pH. EDTA starts solubilizing at around pH 7 and is completely soluble at pH 8. Make up the volume to 100 ml with Milli Q water. | ||
| EGTA (100 mM; pH 7.4) (For 100 mL) | Molecular Weight: 380.4 Add 3.804 g EGTA in 50 ml Milli Q water and check the pH (pH~3) Add 10 N NaOH dropwise while stirring and monitor the pH EGTA becomes soluble at pH 7.0 (approx) Adjust pH to 7.4 Make up the volume to 100 ml with Milli Q water | ||
| FITC Mouse Anti-Human PAC-1 | BD | 340507 | |
| FITC-IETD-FMK Caspase 8 (active) staining kit | Abcam | ab65614 | |
| Mitotracker Red CMXRos (mitochindria labeling dye) | Invitrogen | M7512 | Stock= 1 mM (50 µg dissolved in 90 µl DMSO) Sub-stock= 100 µM (10 µl Stock + 90 µl DMSO) Working concentration= 500nM (0.5 µl in 100 µl) |
| PE Annexin V | BD Pharmingen | 560930 | |
| Prostaglandin E1 | Sigma | P5515 | Stock= 20 mM (1 mg dissolved in 141 µL DMSO) Sub-stock= 1 mM (10 µl Stock + 190 µL DMSO) Working concentration= 3 µM (3 µL in 1 mL) |
| Rhod-2 AM | Invitrogen | R1244 | Stock= 5 mM (1 mg dissolved in 178 µL DMSO) Sub-stock= 100 µM (10 µL Stock + 90 µL DMSO) Working concentration= 500 nM (0.5 µL in 100 µL) |
| Thrombin from human plasma | Sigma | T7572 |
References
- Munnix, I., Cosemans, J., Auger, J., Heemskerk, J. Platelet response heterogeneity in thrombus formation. Thromb Haemost. 102 (12), 1149-1156 (2009).
- Heemskerk, J. W. M., Mattheij, N. J. A., Cosemans, J. M. E. M. Platelet-based coagulation: Different populations, different functions. J Thromb Haemost. 11 (1), 2-16 (2013).
- Podoplelova, N. A., et al. Coagulation factors bound to procoagulant platelets concentrate in cap structures to promote clotting. Blood. 128 (13), 1745-1755 (2016).
- Mann, K. G., Butenas, S., Brummel, K. The dynamics of thrombin formation. Arterioscler Thromb Vasc Biol. 23 (1), 17-25 (2003).
- Jackson, S. P., Schoenwaelder, S. M. Procoagulant platelets: Are they necrotic. Blood. 116 (12), 2011-2018 (2010).
- Hua Vivien Mun Yee, V. M. C. Procoagulant platelets and the pathways leading to cell death. Semin Thromb Hemost. 41 (04), 405-412 (2015).
- Dale, G. L. Coated-platelets: An emerging component of the procoagulant response. J Thromb Haemost. 3 (10), 2185-2192 (2005).
- Vulliamy, P., Armstrong, P. C. Platelets in hemostasis, thrombosis, and inflammation after major trauma. Arterioscler Thromb Vasc Biol. 44 (3), 545-557 (2024).
- Vulliamy, P., et al. Histone H4 induces platelet ballooning and microparticle release during trauma hemorrhage. Proc Natl Acad Sci U S A. 116 (35), 17444-17449 (2019).
- Aliotta, A., Bertaggia Calderara, D., Zermatten, M. G., Marchetti, M., Alberio, L. Thrombocytopathies: Not just aggregation defects-the clinical relevance of procoagulant platelets. J Clin Med. 10 (5), 894 (2021).
- Kirkpatrick, A. C., Vincent, A. S., Dale, G. L., Prodan, C. I. Coated platelets predict stroke at 30 days following TIA. Neurology. 89 (2), 125-128 (2017).
- Kirkpatrick, A. C., Tafur, A. J., Vincent, A. S., Dale, G. L., Prodan, C. I. Coated platelets improve prediction of stroke and transient ischemic attack in asymptomatic internal carotid artery stenosis. Stroke. 45 (10), 2995-3001 (2014).
- Suzuki, J., Umeda, M., Sims, P. J., Nagata, S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 468 (7325), 834-838 (2010).
- Prodan, C. I., Vincent, A. S., Dale, G. L. Coated platelet levels correlate with bleed volume in patients with spontaneous intracerebral hemorrhage. Stroke. 41 (6), 1301-1303 (2010).
- Josefsson, E. C., et al. Consensus report on markers to distinguish procoagulant platelets from apoptotic platelets: Communication from the Scientific and Standardization Committee of the ISTH. J Thromb Haemost. 21 (8), 2291-2299 (2023).
- Kulkarni, P. P., Sonkar, V. K., Gautam, D., Dash, D. AMPK inhibition protects against arterial thrombosis while sparing hemostasis through differential modulation of platelet responses. Thromb Res. 196, 175-185 (2020).
- Ekhlak, M., et al. Necroptosis executioner MLKL plays pivotal roles in agonist-induced platelet prothrombotic responses and lytic cell death in a temporal order. Cell Death Differ. 30 (8), 1886-1899 (2023).
- Kholmukhamedov, A., Janecke, R., Choo, H. -. J., Jobe, S. M. The mitochondrial calcium uniporter regulates procoagulant platelet formation. J Thromb Haemost. 16 (11), 2315-2321 (2018).
- Choo, H. -. J., Saafir, T. B., Mkumba, L., Wagner, M. B., Jobe, S. M. Mitochondrial calcium and reactive oxygen species regulate agonist-initiated platelet phosphatidylserine exposure. Arterioscler Thromb Vasc Biol. 32 (12), 2946-2955 (2012).
- Jobe, S. M., et al. Critical role for the mitochondrial permeability transition pore and cyclophilin D in platelet activation and thrombosis. Blood. 111 (3), 1257-1265 (2008).
- Leytin, V., Allen, D. J., Mykhaylov, S., Lyubimov, E., Freedman, J. Thrombin-triggered platelet apoptosis. J Thromb Haemost. 4 (12), 2656-2663 (2006).
- Lopez, J. J., Salido, G. M., Pariente, J. A., Rosado, J. A. Thrombin induces activation and translocation of Bid, Bax and Bak to the mitochondria in human platelets. J Thromb Haemost. 6 (10), 1780-1788 (2008).
- Wolf, B. B., et al. Calpain functions in a caspase-independent manner to promote apoptosis-like events during platelet activation. Blood. 94 (5), 1683-1692 (1999).
- Kim, O. V., et al. Fatal dysfunction and disintegration of thrombin-stimulated platelets. Haematologica. 104 (9), 1866-1878 (2019).
- Schoenwaelder, S. M., et al. Two distinct pathways regulate platelet phosphatidylserine exposure and procoagulant function. Blood. 114 (3), 663-666 (2009).
- Abbasian, N., Millington-Burgess, S. L., Chabra, S., Malcor, J. -. D., Harper, M. T. Supramaximal calcium signaling triggers procoagulant platelet formation. Blood Adv. 4 (1), 154-164 (2020).
- Le, T., et al. An inner activation gate controls TMEM16F phospholipid scrambling. Nat Commun. 10 (1), 1846 (2019).
- Yang, H., et al. TMEM16F forms a Ca2+-activated cation channel required for lipid scrambling in platelets during blood coagulation. Cell. 151 (1), 111-122 (2012).
- Mattheij, N. J. A., et al. Dual mechanism of integrin closure in procoagulant platelets. J Biol Chem. 288 (19), 13325-13336 (2013).
- Liu, F., et al. Mitochondrially mediated integrin αiIbβ3 protein inactivation limits thrombus growth. J Biol Chem. 288 (42), 30672-30681 (2013).
- van Kruchten, R., et al. Both TMEM16F-dependent and TMEM16F-independent pathways contribute to phosphatidylserine exposure in platelet apoptosis and platelet activation. Blood. 121 (10), 1850-1857 (2013).
- Goelz, N., et al. Platelets express adaptor proteins of the extrinsic apoptosis pathway and can activate caspase-8. PLOS ONE. 16 (1), e0244848 (2021).
- Shlomovitz, I., Speir, M., Gerlic, M. Flipping the dogma - phosphatidylserine in non-apoptotic cell death. Cell Commun Signal. 17 (1), 139 (2019).
- Nadine, J. A. M., et al. Coated platelets function in platelet-dependent fibrin formation via integrin αIIbβ3 and transglutaminase factor XIII. Haematologica. 101 (4), 427-436 (2016).
- Michelson, A. D. Platelet activation by thrombin can be directly measured in whole blood through the use of the peptide GPRP and flow cytometry: Methods and clinical applications. Blood Coagul Fibrinolysis. 5 (1), 121-131 (1994).
- Taylor, M. R., Couto, J. R., Scallan, C. D., Ceriani, R. L., Peterson, J. A. Lactadherin (formerly BA46), a membrane-associated glycoprotein expressed in human milk and breast carcinomas, promotes Arg-Gly-Asp (RGD)-dependent cell adhesion. DNA Cell Biol. 16 (7), 861-869 (1997).
- Andersen, M. H., Graversen, H., Fedosov, S. N., Petersen, T. E., Rasmussen, J. T. Functional analyses of two cellular binding domains of bovine lactadherin. Biochemistry. 39 (20), 6200-6206 (2000).
- Tan, C. W., Bourcy, M., Pasalic, L., Chen, V. M. Flow cytometry assessment of procoagulant platelets using a dithiol-reactive probe functional disulfide bonds: Methods and protocols. Methods Mol Biol. 1967, 305-321 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved