A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Validated Immunochemical Assay for Comprehensive Determination of the Human Epidermal Growth Factor Receptor 2 Released from and Bound to Cells
In This Article
Summary
We present a validated sandwich ELISA assay using novel anti-HER2 monoclonal antibodies. This assay enables precise quantification of cell-bound and released HER2 protein from in vitro cultured cells and other samples, including blood and tissues.
Abstract
Human epidermal growth factor receptor 2 (HER2) is a well-established cancer marker. It became a very successful diagnostic and therapeutic target, especially in breast cancer and other HER2-expressing cancer types. In the clinic, the gold-standard immunohistochemical diagnostic methods employing the specific anti-HER2 antibodies are used to measure the expression level of the membrane-bound receptor. The soluble extracellular domain (ECD) of HER2 that is released from the overexpressing cells circulates in the blood and can reflect the tissue expression of the receptor. There is a need for accurate and validated assays to correlate the concentration of the circulating HER2 protein with disease clinical manifestations.
Our team has developed and validated the novel sandwich enzyme-linked immunosorbent assay (ELISA) for quantification of the membrane-bound and the released from cells ECD domain of HER2. The assay uses two unique monoclonal antibodies specific to HER2 developed previously. The quantitation range includes HER2 concentration from 1.56-100 ng/mL, which is expected for cancer cells cultured in vitro and shows sensitivity at the level of 0.5 ng/mL. The satisfactory intra- and inter-assay precision and accuracy of the method make it applicable for HER2 quantification in various types of biological samples, including cell culture medium, serum, and solid tumor tissue. Here, we focus on the comprehensive determination of the receptor-associated and secreted by the in vitro cultured cancer cells. The paper presents a step-by-step protocol for the quantification of HER2 protein that can be employed for testing a variety of cell lines, blood, and tissues.
Introduction
The success of modern therapeutics often relates to precision medicine that is based on accurate identification of therapy-sensitive patients1. Among these therapies are the anti-HER2 drugs targeting the receptor overexpressed on a variety of tumors, including breast, endometrium, stomach, lung, and others. Several HER2-targeting agents are available with confirmed benefits in patients with HER2-positive cancers, including HER2-low type2. Confirmation of the HER2-positive status is critical for the identification of the potential responding patients; however, it remains a challenge, especially in the HER2-low group.
The gold standard methods in clinical settings, routinely used for HER2 testing, include immunohistochemistry (IHC) protein expression and HER2 gene amplification by fluorescence in situ hybridization (FISH) approaches. Additionally, the Oncotype DX assay is used for HER2 mRNA expression. Tissue biopsy required for these methods makes the determination of patient eligibility for appropriate treatment and their potential responsiveness to therapies uncertain. Despite the updated 2018 guidelines by the American Society of Clinical Oncology (ASCO) and the College of American Pathologists (CAP) to reduce the variability between the testing units, HER2 concordance remains a subject for improvement3.
HER2 is a proto-oncogene member of the epidermal growth factor receptor (EGFR) family that overexpression and activation in the pathological states result in an aggressive outcome or contributes to a poor prognosis4. HER2 is a 185 kDa modular protein anchored in the cell membrane that contains a cytoplasmic tyrosine kinase and an extracellular domain (ECD).The HER2 ECD can be shed from cells to be released into the extracellular matrix5 as the cell-free protein, further circulating in the blood. Increased HER2 expression might be reflected by the higher level of the circulating ECD, presenting a valuable predictive and prognostic marker6,7, a surrogate marker of the treatment response8, or as a complementary method to IHC to identify patients eligible for anti-HER2 treatment9. However, the challenge remains in establishing the correlation between the tumor HER2 expression and the systemic level of the receptor in blood that could have a clinical meaning.
The released HER2 ECD can be quantified using Enzyme-linked immunosorbent assay (ELISA)10. The sandwich ELISA is an approach that employs two specific antibodies binding different epitopes on the same target antigen. It enables accurate measurement of solubilized proteins in the easily accessible blood- and liquid-based biological material (so-called liquid biopsy). Despite the presence of Food and Drug Administration (FDA)-approved assays, there is controversy over the utility of the HER2 ECD diagnostics5 and the cut-off value for an increased HER2 level in blood. More research with the validated methods and uniformly accepted thresholds is needed to confirm the applicability of the assays11.
The critical components of any ELISA are capturing (immobilized on the plate and defining the assay specificity and sensitivity) and detecting antibodies (added after the samples have been applied) antibody (Figure 1). In this report, we present the ELISA protocol based on the recently developed new anti-HER2 ECD monoclonal antibodies (mAb) that were generated, purified on an affinity column, and thoroughly characterized, and present unique sequences12. The developed ELISA that employs these custom antibodies shows its utility for accurate quantification of the HER2 protein associated with the cell membrane and released into a culture medium for comprehensive assessment of the receptor status. The assay can be utilized in preclinical testing and to support ongoing research. The performance of the assay has been further tested on biological samples of different origins, including serum and tissue homogenates12, to show potential in the development of research, diagnostics, and novel anti-HER2 treatments in the future.
Protocol
1. Culturing of human cancer cells
- Culture MDA-MB-231, SK-BR-3, and SK-OV-3 lines in Dulbecco's Modified Eagle's medium containing 4.5 g/L of ᴅ-glucose (DMEM-HG), supplemented with 2 mM ʟ-glutamine and 10% (v/v) heat-inactivated FBS. Incubate cultures at 37 °C in a humidified atmosphere of 5% CO2.
- When the culture reaches ~80% confluence, detach cells by trypsinization and collect them into separate 15 mL conical tubes.
- Count the cells with an automatic cell counter and seed cells for the experiment into 12-well plates at a density of 3 × 105 cells/per well in 1 mL of growth medium. Incubate cultures at 37 °C in a humidified atmosphere of 5% CO2.
NOTE: Seed one well per each time point of the experiment. Both post-culture medium and cell lysates will be used in further analysis.
2. Sample collection and preparation
- From the 24 h culture, collect culture medium from one well of each cell line into a 1.5 mL tube.
- Centrifuge the collected samples at 10 000 × g for 10 min at 4 °C. Transfer the supernatant to a new tube and store at -20 °C to further determine the secreted ECD HER2 level.
- Wash the remaining cells on the plate with 1 mL of cold PBS, aspirate gently, and discard the PBS.
- Add 200 µL of RIPA buffer (supplemented with 1% of protease inhibitor) into each well and incubate for 5 min. Scrape the cells with the cell scraper and mix by pipetting several times to homogenate the lysate.
- Collect the lysed cells into a new tube and slowly aspirate into 2 mL syringe with a needle (G21 / 0.8 mm × 40 mm). Repeat the aspiration step 5-10 times to further disintegrate cells.
- Centrifuge the collected samples at 10 000 x g for 10 min at 4 °C. Transfer the supernatant to a new tube and store at -20 °C for further determination of cell-bound fraction of HER2 protein.
- Repeat collection of culture medium and cellular materials, following the preparation steps for the remaining time points - 48 h and 72 h of culture.
3. Performing a sandwich ELISA for HER2 determination
- Prepare the buffers.
- Coating buffer: Prepare 0.1 M NaHCO3 solution by dissolving 0.42 g of NaHCO3 in 50 mL of distilled water and mix thoroughly. Adjust pH to 9.6 with 3 M NaOH.
- Blocking buffer: Prepare 5% non-fat dairy milk (NFDM) in PBS by adding 0.5 g of blotting milk powder to 10 mL of PBS and mix thoroughly.
- Washing buffer: Prepare phosphate-buffered saline with Tween 20 (PBST) by adding 0.5 mL of 0.05% Tween-20 to 1000 mL of PBS and mix (avoid excessive foaming).
- Biotinylate the detecting antibody.
- Biotinylate 50-200 µg of detecting anti-HER2 antibody (clone 70.21.73.67) using a commercial biotin labeling kit according to the manufacturer's instructions.
NOTE: Reliability and repeatability of the biotinylation process are based on the measurement of the final protein concentration of the resultant antibody and the batch-to-batch comparison of the product.
- Biotinylate 50-200 µg of detecting anti-HER2 antibody (clone 70.21.73.67) using a commercial biotin labeling kit according to the manufacturer's instructions.
- Coat the plate.
- Dilute the anti-HER2 capturing antibody (clone 70.27.58) to the concentration of 1 µg/mL in the coating buffer. To coat one plate, use 6.5 mL of the capturing antibody solution.
- Using a multi-channel pipette, add 100 µL of the capturing antibody solution (prepared in step 3.3.1) to each well on a 96-well ELISA plate.
NOTE: Avoid the outermost wells of the plate (e.g., A1, H12) to reduce variability caused by the edge effect. The outermost wells should be filled with distilled water to ensure stable reaction conditions. - Cover the plate with a sealing film.
- Incubate plate overnight (O/N) at 4 °C.
- After O/N incubation, place the plate at room temperature (RT) on a horizontal microplate shaker (20-30 rpm with a tilt angle of 4°-6°) for 1 h.
- Using a microplate washer, aspirate the coating solution and wash the plate three times with a washing buffer (3 x 300 µL of PBST per well).
- Block the plate .
- Using a multi-channel pipette, add 100 µL of blocking buffer (5% NFDM in PBS) to each coated well to block nonspecific binding sites.
- Cover the plate with a sealing film.
- Incubate the plate for 1 h at 37 °C in a humidity chamber.
- Using a microplate washer, aspirate the coating solution and wash the plate three times with a washing buffer (3 x 300 µL of PBST per well).
- Prepare a calibration curve and positive control (Figure 2).
- Prepare the working standard solution of HER2 antigen by adding 2 µL of 1 mg/mL stock solution to a 1998 µL of PBS to obtain 0.001 mg/mL (1000 ng/mL) HER2 concentration.
- Dilute the working standard solution (WSS) by adding 200 µL of the solution to 1800 µL of PBS to obtain the standard solution 1 (STD 1).
- Prepare subsequent six standards (STD 2-STD 7) by serial dilutions of STD 1 in PBS (Figure 2).
- STD 2: Add 500 µL of STD 1 to 500 µL of PBS and mix thoroughly to prepare 50 ng/mL.
- STD 3: Add 500 µL of STD 2 to 500 µL of PBS and mix thoroughly to prepare 25 ng/mL.
- STD 4: Add 500 µL of STD 3 to 500 µL of PBS and mix thoroughly to prepare 12.5 ng/mL.
- STD 5: Add 500 µL of STD 4 to 500 µL of PBS and mix thoroughly to prepare 6.25 ng/mL.
- STD 6: Add 500 µL of STD 5 to 500 µL of PBS and mix thoroughly to prepare 3.125 ng/mL.
- STD 7: Add 500 µL of STD 6 to 500 µL of PBS and mix thoroughly to prepare 1.5625 ng/mL.
- STD 8: Add 500 µL of PBS to prepare 0 ng/mL.
- Positive control: Dilute STD 1 by adding 100 µL of the STD 1 solution to 900 µL of PBS to obtain a solution of HER2 at 10 ng/mL protein concentration.
- Prepare samples for spike and recovery experiments.
- Prepare the matrix samples (PBS, cell lysates, and culture medium) spiked with the known HER2 protein concentration. Dilute cell lysates 1:2000 by adding 2.5 µL of appropriate cell lysate to 5 mL of PBS to acquire a sample matrix with HER2 level below LOD of the method.
- Use WSS (1000 ng/mL) and STD1 (100 ng/mL) to spike matrix samples.
- For each matrix, prepare 6 samples with concentrations of 0, 2, 5, 10, 30, and 50 ng/mL.
- To prepare 0 ng/mL, add 400 µL of PBS/culture medium/cell lysate.
- To prepare 2 ng/mL, add 8 µL of STD1 to 392 µL of PBS/culture medium/cell lysate and mix thoroughly.
- To prepare 5 ng/mL, add 20 µL of STD1 to 380 µL of PBS/culture medium/cell lysate and mix thoroughly.
- To prepare 10 ng/mL, add 4 µL of WSS to 396 µL of PBS/culture medium/cell lysate and mix thoroughly.
- To prepare 30 ng/mL, add 12 µL of WSS to 388 µL of PBS/culture medium/cell lysate and mix thoroughly.
- To prepare 50 ng/mL, add 20 µL of WSS to 380 µL of PBS/culture medium/cell lysate and mix thoroughly.
- Add the sample, blank, and standard.
- Using a single-channel pipette, add 100 µL of standards, blank (PBS), and tested samples (cell lysates and culture medium) to appropriate wells.
NOTE: It is advised to run each sample in triplicate. - Cover the plate with a sealing film.
- Incubate the plate for 1 h at 37 °C in a humidity chamber.
- Using a microplate washer, aspirate the coating solution and wash the plate three times with a washing buffer (3 x 300 µL of PBST per well).
- Using a single-channel pipette, add 100 µL of standards, blank (PBS), and tested samples (cell lysates and culture medium) to appropriate wells.
- Detect antibody binding.
- Dilute the biotinylated detecting antibody (clone 70.21.73.67) in PBS to a working concentration of 1 µg/mL. For one plate, use 6.5 mL of detecting antibody working solution.
- Using a multi-channel pipette, add 100 µL of the detecting antibody working solution to each well.
- Cover the plate with a sealing film.
- Incubate the plate for 1 h at 37 °C in a humidity chamber.
- Using a microplate washer, aspirate the coating solution and wash the plate three times with a washing buffer (3 x 300 µL PBST per well).
- Add Avidin-HRP conjugate.
- Dilute the commercially available Avidin-HRP conjugate 1:40,000 by adding 1 µL of enzymatic conjugate to 40 mL of PBST.
- Using a multi-channel pipette, add 100 µL of the diluted conjugate to each well.
- Cover the plate with a sealing film.
- Incubate the plate for 1 h at 37 °C in a humidity chamber.
- Using a microplate washer, aspirate the coating solution and wash the plate three times with a washing buffer (3 x 300 µL of PBST per well).
- Colorimetric reaction
- Using a multi-channel pipette, add 100 µL of 3,3′,5,5′-Tetramethylbenzidine (TMB) substrate to each well.
- Cover the plate with a sealing film.
- Incubate the plate for 1-5 min at 37 °C away from light and monitor the color development.
- When the color reaches the expected level, add 100 µL of stop solution to terminate the reaction.
NOTE: The expected level corresponds to an intense blue color in wells containing samples with a high concentration of the analyte.
- Acquire and analyze data.
- Measure the absorbance at 450 nm using a microplate reader.
- Using a 4-parametric calibration curve, calculate the sample concentration based on the curve equation.
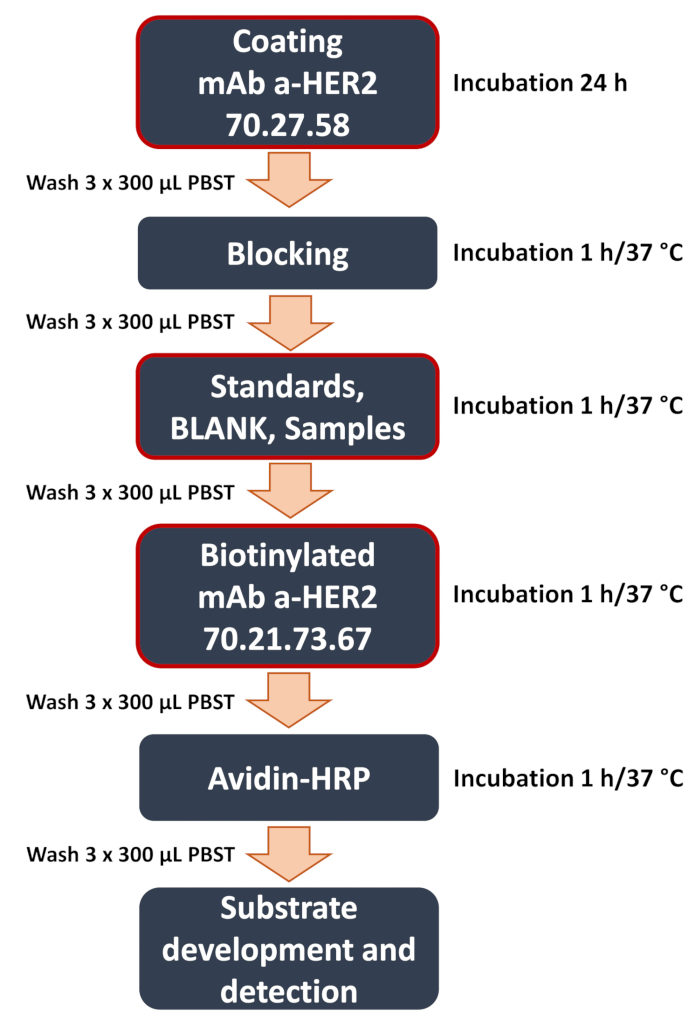
Figure 1: Schematic diagram of developed anti-HER2 sandwich ELISA workflow. Overview of the key steps in the sandwich ELISA procedure. These include the critical steps (highlighted with a red frame) like plate coating with the capturing anti-HER2 antibody 70.27.58, the addition of samples (curve standards, blank, experimental samples of the cell lysates or culture medium), and binding of the detecting anti-HER2 antibody 70.21.73.67. The assay concludes with signal detection and data analysis, where the colorimetric signal is quantified using a microplate reader to determine the concentration of the target antigen. Please click here to view a larger version of this figure.
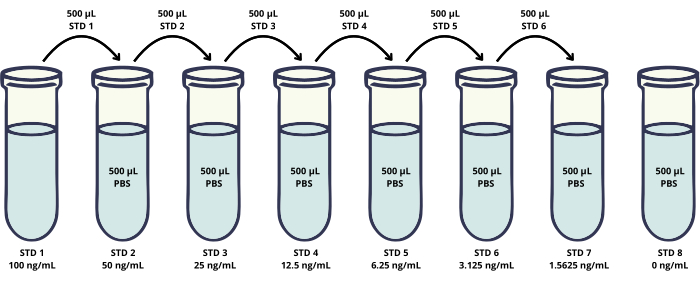
Figure 2: Scheme presenting preparation of the calibration curve standard solutions. Serial dilutions of the HER2 recombinant protein are prepared to generate a calibration curve in a concentration range of 1.56-100 ng/mL (vials STD 1-STD 7). In addition, the negative control sample without the HER2 protein is included (STD 8). Please click here to view a larger version of this figure.
Results
Sandwich ELISA validation
The newly developed assay requires a validation procedure. The important validation parameters include linearity, precision, and detection limits, i.e., lower limit of detection (LLOD) and upper limit of detection. In the previous paper, we have performed thorough method validation. ELISA linearity was tested by using the mocked samples for low (2, 5, 10 ng/mL), medium-high (30 ng/mL), and significantly increased (50 ng/mL) concentrations of the antigen diluted in PBS and ...
Discussion
Among the critical components in constructing a sandwich ELISA, there are capturing antibodies that are immobilized on the plate and contribute to the assay specificity and sensitivity. In the presented assay, we have employed as the capturing antibody the novel monoclonal protein (HER2/70.27.58) generated and characterized in-house. The antibody had a unique sequence of the CDR (complementarity-determining region), and based on the affinity, it presented a sensitivity of ED50 at 0.0922 nM12. The ...
Disclosures
D.L., A.A., A.M., M.S. declare financial support from SDS Optic S.A.; A.A, A.M., M.S. declare SDS Optic S.A. stock ownership.
Acknowledgements
The study was supported by a funds from the National Centre for Research and Development grant STRATEGMEDII/269364/5/NCBR/2015 and EU, Horizon 2020 SME Instrument grant No. 783818.
Materials
| Name | Company | Catalog Number | Comments |
| Biotin labeling kit-NH2 | Abnova | KA0003 | |
| Blotting Grade, powdered milk, low in fat | Roth | T145.1 | |
| Cell Counting Slides for TC10/TC20 cell Counter, Dual-Chamber | Bio-Rad | 145-0011 | |
| Cell Culture Plates | Biologix | 07-6012 | |
| Cell Scrapers | Biologix | 70-1250 | |
| Centrifuge | Ohaus | 30130868 | |
| Class II Biological Safety Cabinet - Telstar Bio II Advance 6 | Telstar | N/A | |
| Clear Flat-Bottom 96-Well Plates | Thermo Fisher | 442404 | |
| Culture Safe CO2 Incubators - Touch 190S | Leec | N/A | |
| Dimethyl sulfoxide | Sigma Aldrich | D2650 | |
| DMEM - high glucose | Sigma Aldrich | D0822 | |
| ELISA plate reader | BioTek | 800TSUVI | |
| FBS Standard, fetal bovine serum | PAN Biotech | P30-19375 | |
| Forced circulation laboratory dryer | BINDER | 9090-0018 | |
| HRP-Avidin | Thermo Fisher | 43-4423 | |
| Human Her2 / ErbB2 Protein, Fc Tag, premium grade | AcroBIOSYSTEMS | HER2-H5253 | |
| Immunowash Microplate Washer | Bio-Rad | 170-7009 | |
| L-Glutamine solution | Sigma Aldrich | G7513 | |
| mAb a-HER2 (clone 70.21.73.67) | SDS Optic | BIO-ABH-2 | |
| mAb a-HER2 (clone 70.27.58) | SDS Optic | BIO-ABH-1 | |
| MDA-MB-231 Cell line | ATCC | HTB-26 | |
| NaHCO3 | POCH | 810530115 | |
| NaOH | POCH | BA0981118 | |
| Protease Inhibitor Cocktail | Sigma Aldrich | P8340 | |
| RIPA Buffer | Sigma Aldrich | R0278 | |
| ROTI Fair PBS | Roth | 1111.2 | |
| SK-BR-3 [SKBR3] Cell line | ATCC | HTB-30 | |
| SK-OV-3 [SKOV-3; SKOV3] Cell line | ATCC | HTB-77 | |
| Stop solution 1x | Abcam | ab210900 | |
| TC20 Automated Cell Counter | Bio-rad | 1450102 | |
| TMB substrate 1x | Abcam | ab210902 | |
| Tween-20 | Sigma Aldrich | P9416 | |
| Vortex | Ohaus | 30392117 | |
| Wave motion shaker | Ohaus | 30391968 |
References
- Subbiah, V., Kurzrock, R. Debunking the delusion that precision oncology is an illusion. Oncologist. 22 (8), 881-882 (2017).
- Swain, S. M., Shastry, M., Hamilton, E. Targeting HER2positive breast cancer: advances and future directions. Nat Rev Drug Discov. 22 (2), 101-126 (2023).
- McLemore, L. E. et al. HER2 testing in breast cancers: comparison of assays and interpretation using ASCO/CAP 2013 and 2018 guidelines. Breast Cancer Res Treat. 187 (1), 95-104 (2021).
- Iqbal, N., Iqbal, N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. 2014, 852748 (2014).
- Tsé, C., Gauchez, A. S., Jacot, W., Lamy, P. J. HER2 shedding and serum HER2 extracellular domain: biology and clinical utility in breast cancer. Cancer Treat Rev. 38 (2), 133-142 (2012).
- Reix, N. et al. A prospective study to assess the clinical utility of serum HER2 extracellular domain in breast cancer with HER2 overexpression. Breast Cancer Res Treat. 160 (2), 249-259 (2016).
- Di Gioia, D. et al. Serum HER2 supports HER2testing in tissue at the time of primary diagnosis of breast cancer. Clin Chim Acta. 430, 86-91 (2014).
- Carney, W. P. et al. Monitoring the circulating levels of the HER2/neu oncoprotein in breast cancer. Clin Breast Cancer. 5 (2), 105-116 (2004).
- Azar, F. P., Fatemeh, H. S. Value of serum human epidermal growth factor receptor 2 (HER2)/neu testing in breast cancer patients to maximize detection of HER2/neupositive patients and susceptibility to trastuzumab. Clin Biochem. 44 (13) (2011).
- Pandey, I., Misra, V., Pandey, A., Verma, A. Expression of HER2/neu in gastric adenocarcinoma and its correlation with serum HER2/neu level and Ecadherin expression. Indian J Pathol Microbiol. 65 (1), 35-41 (2022).
- Agnon, V. et al. ELISA assay employing epitopespecific monoclonal antibodies to quantify circulating HER2 with potential application in monitoring cancer patients undergoing therapy with trastuzumab. Sci Rep. 10 (1), 3016 (2020).
- Antos, A. et al. The unique monoclonal antibodies and immunochemical assay for comprehensive determination of the cellbound and soluble HER2 in different biological samples. Sci Rep. 14 (1), 3978 (2024).
- Andreasson, U. et al. A practical guide to immunoassay method validation. Front Neurol. 6, 179 (2015).
- Perrier, A., Gligorov, J., Lefèvre, G., Boissan, M. The extracellular domain of HER2 in serum as a biomarker of breast cancer. Lab Invest. 98 (6), 696-707 (2018).
- Van Gorkom, T., van Arkel, G. H. J., Voet, W., Thijsen, S. F. T., Kremer, K. Consequences of the edge effect in a commercial enzymelinked immunosorbent assay for the diagnosis of Lyme neuroborreliosis. J Clin Microbiol. 59 (8), e0328020 (2021).
- Sadok, I., Rachwał, K., Staniszewska, M. Simultaneous quantification of selected kynurenines analyzed by liquid chromatographymass spectrometry in medium collected from cancer cell cultures. J Vis Exp. 159, 61031 (2020).
- Giordani, E. et al. Monitoring changing patterns in HER2 addiction by liquid biopsy in advanced breast cancer patients. J Exp Clin Cancer Res. 43 (1), 182 (2024).
- Wu, Y. et al. Imaging and monitoring HER2 expression in breast cancer during trastuzumab therapy with a peptide probe 99mTcHYNICH10F. Eur J Nucl Med Mol Imaging. 47 (11), 2613-2623 (2020).
- NinioMany, L. et al. miR125a induces HER2 expression and sensitivity to trastuzumab in triplenegative breast cancer lines. Front Oncol. 10, 191 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved