Method Article
Isolation, Fixation and Characterization of Juvenile Gilthead Seabream Head Kidney Leukocytes by Flow Cytometry
In This Article
Summary
This manuscript describes the isolation and fixation of leukocytes extracted from gilthead seabream's head-kidney and the assessment of their viability by flow cytometry. This work contributes to the standardization of protocols and leverages the processing of a higher number of samples without compromising sample quality, promoting advancements in fish immunology knowledge.
Abstract
Immunity is crucial for the physiological regulation of organisms, serving as the primary defense against pathogens and environmental stressors. The isolation and analysis of immune cells provide key insights into immune responses to external pressures. However, the lack of harmonized protocols for less studied species, such as marine fish, often leads to technical and analytical challenges that hamper data interpretation and a thorough understanding of species-specific immune responses. This study aimed to set up an optimized flow cytometry-based analytical procedure to characterize and determine the viability of leukocytes from the head kidney (the main hematopoietic organ in teleost fish) of juvenile gilthead seabream (Sparus aurata). The procedure began with the isolation of leukocytes through a homogenization process using Hanks' balanced salt solution, followed by an optimized Percoll density gradient centrifugation method to ensure high recovery rates of leukocytes with minimal erythrocyte contamination required for efficient subsequent flow cytometry analysis. Additionally, a novel technique using a cell-reactive dye (LIVE/DEAD Fixable Dead Cell Stain Kit) was employed to distinguish viable from dead cells based on their fluorescent staining patterns. Fixation was achieved with 3.7% formaldehyde, preserving cell morphology, viability, and staining efficiency. Flow cytometry analysis successfully identified three predominant leukocyte populations: lymphocytes, monocytes, and granulocytes. This method not only allowed viability tests but also the accurate differentiation of cell types. The improvement in flow cytometry protocols represents a step forward in fish immunology by increasing the accuracy and efficiency of immune cell analysis. Furthermore, by allowing the fixation of cells for later analysis, this protocol significantly reduces the time and effort required for immune assessments, making it a valuable tool for both research and practical applications in various fields of research.
Introduction
Immunity plays a central role in the physiological regulation of organisms, acting as a primary defense against a wide range of pathogens and environmental stressors1. Like other vertebrates, fish have a complex, dynamic, and coordinated immune system essential for their overall health and well-being1.
Teleost fish possess both innate and adaptive immune systems, which function simultaneously to detect, respond to, and neutralize harmful invaders2. The innate immune system acts as the first line of defense, providing immediate and non-specific responses to pathogens2, while the adaptive immune system develops over time, offering a more specialized response that enables fish to recognize specific pathogens and establish immunological memory3. The fish immune system relies on specialized primary lymphoid organs (i.e., thymus and head kidney) and secondary lymphoid organs (e.g., spleen and mucosa-associated lymphoid tissues (MALT)) to support immune defense and maintain overall health4. The head kidney is the primary hematopoietic organ in teleost fish and plays a crucial role in the development and maturation of immune cells, including leukocytes5.
In recent years, significant progress has been made in studying the immune responses of several fish species2. One key area of focus has been understanding leukocyte populations and their activity. Leukocytes, also known as white blood cells, are generally classified into monocytes, lymphocytes, and granulocytes and play a crucial role in the immune defense of fish. They have phagocytic cells, which are responsible for engulfing and destroying pathogens and release bactericidal reactive oxygen species, contributing to the elimination of invading microorganisms6. Leukocytes are also involved in the inflammatory process, helping to isolate and eradicate infections while promoting tissue repair6. The abundance and activity of leukocyte populations are important indicators of immune status in animal health and disease7,8.
Some studies have demonstrated that stress factors, such as adverse environmental conditions, can alter the number and morphology of erythrocytes and the composition of circulating leukocytes9,10. For instance, as reviewed by Franke et al. (2024), it is crucial to study the immune system of fish in climate change scenarios, as environmental stressors can compromise fish immunity, increase disease susceptibility, and enhance the infectivity of certain pathogens, ultimately accelerating disease progression11. Moreover, understanding fish immunity is essential not only to advance fundamental biological research but also to support various sectors of society, such as the aquaculture industry. As aquaculture continues to expand globally, ensuring the health and welfare of farmed fish species is becoming increasingly important. Yet, fish welfare remains a relatively new area of research, and the immune responses of farmed fish still require thorough and standardized assessments. Prioritizing immune response studies is of utmost importance, as the information acquired can enhance aquaculture's sustainability and productivity through effective and tailored approaches that improve the welfare and resilience of farmed animals.
Leukocyte quantification and identification are usually performed using hematological methods, such as manual counting with Bürker, Neubauer, or Thoma hemocytometers, as well as stained blood smears7,10. To aid in the visualization and differentiation of blood cells, staining kits, such as Wright, May-Grünwald-Giemsa, and Hemacolor, are often employed7,12. However, these manual cell counting techniques are tedious, time-consuming, and prone to human error8,10. Common sources of error include inadequate mixing or dilution of the blood, staining issues, and incorrect loading of the hemocytometer chamber, all of which can lead to inaccurate cell counts12. Furthermore, manual hematological analysis requires a high level of expertise and experience to ensure the reliability and reproducibility of the results7. As the demand for precise and efficient diagnostic tools increases, the development of innovative methods that provide an in-depth understanding of the immune status of fish populations becomes an increasingly important step in advancing this field.
Flow cytometry has emerged as a powerful tool in this context, offering a high-throughput, quantitative approach to analyzing leukocyte populations and cell viability8. This modern diagnostic technology allows for the rapid detection, count, and characterization of individual cells in mixed populations with remarkable precision13. Moreover, flow cytometry allows simultaneous multiparametric measurements for both phenotypic and functional characterization. Although widely used in human clinical settings and veterinary medicine, its application in the study of fish leukocytes remains very limited8. While some research has been conducted on different fish species1,6,8,13,14,15,16,17, several critical challenges still need to be addressed. One major challenge in these analyses is the necessity to obtain suspensions of live leukocytes extracted from peripheral blood or lymphoid tissues, such as the head kidney1. Isolation of leukocytes is often difficult due to a unique characteristic of teleost fish: the presence of nucleated erythrocytes. The unintentional contamination with erythrocytes can interfere with leukocyte analysis due to their size, ovoid shape, and the presence of a nucleus1. It is, therefore, imperative to eliminate erythrocytes from leukocyte suspensions to achieve high leukocyte purity and to study the phenotypic and functional characteristics of leukocytes by flow cytometry analysis. In mammals, leukocyte isolation typically involves erythrocyte osmotic lysis or density gradient separation with Ficoll or Percoll1. However, osmotic lysis is ineffective for both marine and freshwater fish due to their nucleated erythrocytes, which cannot be properly lysed1. Instead, density gradient separation is preferred for fish as it effectively preserves cell stability over time1. Although some studies have successfully isolated leukocytes from juvenile fish, much research still focuses mainly on adult populations17. Nonetheless, early-life stages are not only more vulnerable to disease outbreaks but also smaller in size, making the sampling process more complex and challenging. Another limitation is that current methods are often restricted to a limited number of samples or replicates at a time, as evaluating leukocyte viability requires immediate processing. Delays in sample processing can adversely affect cellular viability, thereby introducing further complications into the sampling process and potentially jeopardizing the entire work.
To the best of our knowledge, none of the published methods have successfully fixed leukocyte cells for subsequent viability analysis by flow cytometry. The present study is pioneering, as it establishes an efficient method for isolating leukocytes from the head kidney of juvenile gilthead seabream (Sparus aurata), the main fish species farmed in southern European countries, using a Percoll density gradient separation methodology. We also present an improved staining-based technique that discriminates live from dead cells while identifying the major leukocyte populations (lymphocytes, monocytes, and granulocytes) through flow cytometry. The improved protocol entails cell fixation, which enables viable cell analysis up to 1-month post-procedure. The implementation of this flow cytometry protocol has the potential to significantly reduce the time and effort typically required for immune assessments, making it a valuable technique for both research and more practical applications within the aquaculture sector. The application of this methodology provides advantages for analyzing a large number of samples, preserving the cells, and allowing for delayed analysis by flow cytometry. Therefore, it might be very helpful to produce valuable insights into fish immune mechanisms and how cell viability is influenced by different environmental or experimental conditions. Moreover, this viability assay can be integrated with multiparametric phenotypic and functional characterization of specific immune cell populations. This approach enables a more comprehensive analysis of several immunological parameters, linking them directly to the corresponding cell types and providing a clearer understanding of immune responses. These findings could contribute to the development of more effective strategies, including improved approaches for disease management in aquaculture.
Protocol
This protocol must be performed by researchers certified in animal experimentation (EU functions A and B). All procedures related to animal handling and sample collection must comply with the ARRIVE guidelines (Animal Research: Reporting of in vivo Experiments) and adhere to ethical standards for the care and use of animals in accordance with the recommendations of the Federation of European Laboratory Animal Science Associations (FELASA). The present study followed all these standards as well as Portuguese legislation for Laboratory Animal Science (EU Directive 2010/63; Decree-Law no. 113/2013). The research was approved by IPMA's Animal Welfare and Ethics Body (ORBEA, LABVIVOS-002-AquaClimAdapt), overseen by the National Authority for the Use of Live Animals, known as the Directorate-General for Food and Veterinary (DGAV), under the ethical clearance number 20596/25-S.
1. Study model and organisms' maintenance
NOTE: This study was specifically designed for juvenile gilthead seabream (Sparus aurata), with an average weight of 30.0 ± 5.0 g and a total length of 12.0 ± 2.0 cm. This method may not directly apply to other fish species, as different species have unique physiological and immunological characteristics that may affect leukocyte isolation, cell fixation, and viability assessment. Adaptations to the protocol may be required for other species, and preliminary studies are recommended to optimize conditions for each target species.
- Acclimate the fish (Quarantine Period)
- Distribute the fish equally in tanks with a large capacity (e.g., two tanks with a total capacity of 660 L each - see Supplementary Figure 1).
NOTE: The tanks to be used may be part of a Recirculating Aquaculture System (RAS), which allows for efficient water use and improved control over water quality. In an RAS, water is continuously recycled and treated within the system, providing a stable and controlled environment for the fish. This setup helps in maintaining optimal conditions for fish health and growth, ensuring uniform distribution and minimizing stress during the study. - Maintain optimal abiotic conditions.
- Keep the fish in quarantine for 3 weeks under conditions mimicking their natural habitat:
temperature: 20.0 ± 0.5 °C;
dissolved oxygen: 7.2 ± 0.2 mg/L;
salinity: 35.0 ± 0.5 ‰;
pH: 8.0 ± 0.1 units;
photoperiod: 14 h light/10 h dark.
NOTE: Ideal maintenance conditions may vary between different fish species. Other species may have different requirements, so it is important to adapt these conditions to the specific needs of the target species to ensure the health and welfare of the organisms. Additionally, seawater temperature and photoperiod can change with the seasons, so seasonal variations should be considered when replicating natural conditions in the laboratory.
- Keep the fish in quarantine for 3 weeks under conditions mimicking their natural habitat:
- Distribute the fish equally in tanks with a large capacity (e.g., two tanks with a total capacity of 660 L each - see Supplementary Figure 1).
- Initiate the experimental study.
- Define the number of tanks and fish required based on the experimental setup design of each case study.
- After the quarantine period, transfer the fish into independent RAS (see Supplementary Figure 2).
- Equip each system with protein skimmers to remove excessive organic compounds from the water; physical filtration (filter bag [400 µm], filter sponge, and glass wool); biological filtration (bio balls [1.5"], ultraviolet water sterilizer, and submerged air stones); automatic seawater refrigeration systems and submerged digital heaters, both connected to a computerized control system (ProfiLux) with temperature sensors to adjust the temperature in each tank; submerged air stones in each tank to control dissolved oxygen.
- Acclimate the fish for 2 weeks in the new systems before proceeding with the experiment.
- Perform daily maintenance
- Remove fish feces from each incubation tank and perform a 25% seawater renewal.
- Measure temperature using a portable precision thermometer.
- Monitor other seawater abiotic parameters (salinity, dissolved oxygen, and pH) using a multi-parameter measuring instrument.
- Adjust seawater abiotic parameters as necessary to ensure stability throughout the experiment.
NOTE: The abiotic conditions in the experimental systems can vary depending on the specific requirements of each case study. For instance, if the study aims to simulate seasonal changes or marine heat waves, the temperature and photoperiod should be adjusted accordingly to mimic natural seasonal variations. Similarly, if the study focuses on simulating hypoxic conditions or ocean acidification, adjustments should be made to the oxygen levels and pH of the seawater. This ensures the experimental conditions are as realistic and relevant as possible, enhancing the validity and applicability of the study's findings. - Assess the health and well-being of the fish, identifying and managing signs of stress or disease as described in steps 1.2.4.6-1.2.4.8.
- Look for abnormal behaviors such as erratic swimming, loss of appetite, lethargy, aggression, or isolation.
- Check for signs of disease, including lesions, ulcers, discoloration, clamped fins, excessive mucus, or rapid gill movement.
- Record all observations, noting the date and actions taken.
- Conduct weekly water quality tests.
- Measure ammonia (NH3/NH4), nitrite (NO2-), and nitrate (NO3-) levels using colorimetric tests.
NOTE: Ensure that all these parameters are below detectable levels. If levels exceed limits, perform an additional water change, increase aeration, or adjust filtration.
- Measure ammonia (NH3/NH4), nitrite (NO2-), and nitrate (NO3-) levels using colorimetric tests.
- Feed and monitor fish nutrition.
- Provide a high-quality diet that meets the specific nutritional requirements of juvenile fish (see Supplementary Table 1 for an example of the detailed composition of the diet).
NOTE: Ensure that the size of the aquafeed pellets (2-3 mm) is suitable for the juveniles, facilitating ingestion and digestion. - Adjust the feed quantity to correspond to 2% of the fish's average body weight per day.
- Feed the fish manually twice daily - once in the morning and once in the afternoon (set a specific time to maintain a stable feeding routine).
- Provide a high-quality diet that meets the specific nutritional requirements of juvenile fish (see Supplementary Table 1 for an example of the detailed composition of the diet).
2. Fish sampling, euthanasia, dissection, and head kidney collection
- Sample the fish from the tanks.
- Select fish randomly from the tanks to avoid sampling bias.
- Use a net to gently transfer the fish into a temporary holding container filled with tank water.
NOTE: Minimize handling time to reduce stress.
- Prepare the euthanasia solution.
- Use an appropriate container (e.g., a 3 L bucket) suitable for holding the fish.
- Dissolve the appropriate amount of Tricaine (MS-222) in seawater to achieve a final concentration of 200-300 mg/L18 (CAUTION See Supplementary Table 2 and Supplementary File 1).
- Buffer the solution with sodium bicarbonate to a pH of 7.2-7.5.
- Administer the euthanasia solution.
- Place the fish in the euthanasia solution for at least 10 min or until cessation of opercular movement and loss of reflexes are observed (confirm euthanasia by checking for no response to external stimuli).
- Record the fish's body weight (g) and total length (cm).
- Perform fish dissection.
NOTE: To ensure optimal sample quality and cell viability, the dissection process should be completed as quickly as possible, ideally within 5 min after euthanasia.- Ensure that the laboratory temperature is kept at 19 °C using air conditioning to minimize fluctuations during sampling and processing.
- Sterilize the dissection tools (preferably autoclave metal instruments whenever possible) and set up a clean workspace with 70% ethanol (CAUTION, see Supplementary Table 2).
- Place the euthanized fish on its side on a sterile dissection tray with the head pointing toward the non-dominant hand.
- Using a scalpel, make a careful incision along the ventral midline of the fish from the vent (anus) up towards the gills.
NOTE: Be cautious not to cut too deeply to avoid damaging internal organs. - Carefully use dissection scissors to extend the incision and expose the internal organs. Gently lift the body wall flaps to provide a clear view of the internal anatomy.
- Locate the head kidney: The head kidney is positioned just behind the gills, near the anterior dorsal region of the body cavity, and extends along the top side of the body cavity, beneath the vertebral column.
NOTE: The head kidney is typically darker in color compared to the surrounding tissues (Figure 1). - Carefully clear away any surrounding tissues, such as fat and connective tissue, to better visualize the head kidney.
NOTE: This step requires delicate handling to avoid damaging the head kidney. - Using fine-tipped forceps and scissors, carefully lift the head kidney and make precise cuts around it to free it from the surrounding tissues.
NOTE: Handle the tissue gently to prevent damage. In a fish weighing approximately 30 g, the head kidney is expected to weigh around 20-30 mg. - Immediately place the excised organ into a cell strainer (100 µm nylon mesh) within a sterile Petri dish to ensure sterility and to facilitate subsequent processing steps.
NOTE: From this point onwards, all steps should be performed as quickly as possible (within 5 min) to maintain cell viability. To keep the sample cool, perform the following steps with the Petri dish placed on a container filled with ice and covered with aluminum foil.
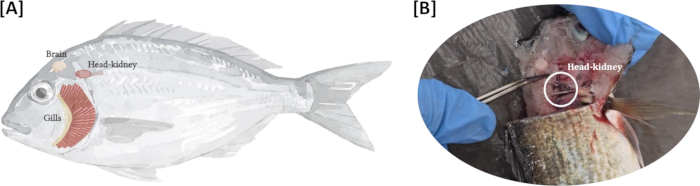
Figure 1: Position of the head kidney: (A) Illustration depicting the typical location of the head kidney behind the gills and along the anterior dorsal region of the body cavity; (B) Representative image of the head kidney in seabream, emphasizing its darker coloration compared to the surrounding tissues. Please click here to view a larger version of this figure.
3. Isolation of head kidney leukocytes (Figure 2)

Figure 2: Illustrative description of leukocyte isolation from the head kidney. The protocol involves several steps: starting with the homogenization of the tissue, followed by density gradient centrifugation, collection of the leukocytes ring, washing of the leukocytes ring, and concluding with the resuspension and adjustment of cell concentration. Please click here to view a larger version of this figure.
- Homogenize the tissue.
- Place 2 mL of Hanks' Balanced Salt Solution (HBSS) in a sterile Petri dish.
- Ensure that the mesh of the cell strainer (100 µm nylon mesh) is in contact with the HBSS but not fully submerged.
- Macerate the head kidney on the cell strainer using the plunger of a syringe. Apply gentle pressure to force the organ fragments through the nylon mesh, creating a cell suspension.
NOTE: If processing multiple samples, the Petri dish containing the cell suspension can be stored in the refrigerator at 4 °C for a few minutes until the next step. This cell suspension will be used for leukocyte isolation.
- Perform density gradient centrifugation.
- Prepare a density gradient medium solution with a density of 1.077 g/mL, osmolarity of 353 mOsm/kg, and pH of 7.4 (see Supplementary File 1).
NOTE: Osmolarity may vary among different fish species, so it is crucial to adjust the osmolarity of the density gradient medium solution to meet the specific requirements of each species. - In 5 mL polystyrene round-bottom tubes, carefully add 600 µL of the density gradient medium solution.
- Take the cell suspension (2 mL) and slowly add it to the tube containing the density gradient medium. The first drop is crucial - add it very gently to avoid destabilizing the density gradient medium phase.
NOTE: This should be done at a 3:10 ratio (Density gradient medium:Cell suspension). Use a sterile 1 mL Pasteur pipette for precise and gentle addition. Ensure the tip of the pipette is touching the side of the tube to prevent disturbing the density gradient medium layer. Avoid mixing the sample with the density gradient medium. - Centrifuge the tubes at 400 x g for 45 min at 4 °C with the brake off. This ensures that the layers remain intact during the process.
NOTE: After centrifugation, distinct layers should be visible. Leukocytes will form a ring at the interface between the density gradient medium and the cellular debris pellet (Figure 3).
- Prepare a density gradient medium solution with a density of 1.077 g/mL, osmolarity of 353 mOsm/kg, and pH of 7.4 (see Supplementary File 1).

Figure 3: Leukocytes ring at the interface between the density gradient medium and the cellular debris pellet. Please click here to view a larger version of this figure.
- Collect the leukocyte ring.
- Using a sterile Pasteur pipette, gently aspirate the leukocyte ring (~100 µL) and transfer it into a 2 mL microcentrifuge tube.
NOTE: Take care not to disturb the layers significantly to avoid contamination. If the leukocyte ring appears to contain debris or dark suspensions, it is advisable to pass the collected leukocyte suspension through a new mini cell strainer (100 µm) to ensure purity. Place the mini cell strainer over the 2 mL microcentrifuge tube and gently transfer the leukocyte suspension through the strainer.
- Using a sterile Pasteur pipette, gently aspirate the leukocyte ring (~100 µL) and transfer it into a 2 mL microcentrifuge tube.
- Wash the leukocyte ring.
- Add HBSS to the microcentrifuge tubes containing the leukocyte ring until the volume reaches 2 mL and gently resuspend the cells.
NOTE: Keep the tubes in a container filled with ice and covered with aluminum foil to maintain the sample at a low temperature. Ensure the tubes do not come into direct contact with the ice. Maintain this cooling setup throughout the washing process. - Centrifuge the samples at 400 × g for 10 min at 4 °C (brake can be on).
- After centrifugation, carefully discard the supernatant, leaving the pellet at the bottom (which may be almost invisible).
- Repeat the washing steps (adding HBSS, resuspending, centrifuging, and discarding the supernatant) until the pellet is free of impurities.
- Add HBSS to the microcentrifuge tubes containing the leukocyte ring until the volume reaches 2 mL and gently resuspend the cells.
- Resuspend and adjust cell concentration.
- Resuspend the cells in 1 mL of HBSS within the same 2 mL microcentrifuge tubes used for the washes.
- Achieve a cell concentration between 1 × 104 and 1 × 106 cells per mL. If the initial pellet is large, additional HBSS may be required to dilute the cell concentration to the desired range. Transfer the cell suspension to a 15 mL tube and add more HBSS as needed based on the cell counts obtained.
NOTE: The use of an automated cell counter is recommended to facilitate the process.
4. Staining and fixation of leukocytes (Figure 4)
NOTE: The LIVE/DEAD Fixable Dead Cell Stain Kits provide an improved method to evaluate cell viability in fixed cells using flow cytometry. These assays utilize a fluorescent reactive dye that interacts with cellular amines. If the cell membranes are compromised, the dye can penetrate the cells, reacting with free amines both inside and on the cell surface, resulting in intense fluorescent staining. Conversely, in viable cells, only the cell-surface amines are available to react with the dye, leading to relatively dim staining. The staining intensity is maintained following fixation with formaldehyde, which also preserves the sample by preventing the growth of microorganisms. The LIVE/DEAD Fixable Dead Cell Stain Kits are identical except for the fluorescent dye - available in blue, violet, aqua, yellow, green, red, far red, or near-IR (infrared). In this study, we used the Near-IR fluorescent reactive dye. Additionally, this single-color assay allows for the testing of other parameters in parallel in a multicolor experiment.
- Prepare the dye.
- Bring the reagents to room temperature (RT): Allow one vial of the fluorescent reactive dye and the vial of anhydrous dimethyl sulfoxide (DMSO) to reach RT before removing the caps.
- Reconstitute the dye: Add 50 µL of DMSO to the vial containing the reactive dye. Mix thoroughly and ensure that the dye has fully dissolved.
- Use the reconstituted dye solution as soon as possible, preferably within a few hours.
NOTE: Each kit includes five individual vials of reactive dye, providing sufficient material to stain at least 40 cell samples. However, after reconstitution, the DMSO solution of the dye is relatively unstable, particularly when exposed to moisture. Any unused portions can be stored for up to 2 weeks at ≤-20 °C, protected from light and moisture.
- Stain and fix the cells.
NOTE: Buffers suitable for cell staining include Hanks' Balanced Salt Solution (HBSS), phosphate-buffered saline (PBS), and Dulbecco's PBS (D-PBS), as long as they do not contain extraneous proteins such as bovine serum albumin or serum. When using an amino-reactive dye, avoid Tris buffers and solutions with sodium azide or extraneous protein for cell resuspension and washing. In this study, we used HBSS due to its balanced ion composition, which helps to maintain osmotic balance and provides essential ions and glucose to support cell metabolism and viability during the staining process. HBSS is also free of extraneous proteins, preventing interference with the reactive dye and ensuring accurate viability assessment.- After counting the cells and adjusting the density to 1 x 106 cells per mL with HBSS, transfer 1 mL of this cell suspension to 2 mL microcentrifuge tubes.
- For each day, induce cell death in at least one sample to be used as a control to set the fluorescence intensity threshold between live and dead cells. Prepare the samples in microcentrifuge tubes as follows:
Tube 1: Live cells - unstained
Tube 2: Live cells - stained
Tube 3: Dead cells - unstained
Tube 4: Dead cells - stained
NOTE: These four tubes only need to be prepared for a single sample in each experiment as controls. For the remaining samples, only two tubes are necessary (Tube 1: sample - unstained and Tube 2: sample - stained), as it will provide information about the cells that were alive and those that were dead.
- For each day, induce cell death in at least one sample to be used as a control to set the fluorescence intensity threshold between live and dead cells. Prepare the samples in microcentrifuge tubes as follows:
- Positive control for cell death: Place the labeled tube Dead unstained (Tube 3) and Dead-stained (Tube 4) in a water bath at 50 °C for 7 min to induce cell death by heat treatment.
- Staining the cells: Add 1 µL of the reconstituted fluorescent reactive dye (from step 4.1.2) to 1 mL of the cell suspension in Tubes 2 and 4 (tubes that will be stained) and mix well.
- Incubate at RT for 30 min, protected from light.
NOTE: If fixation is not required, then you can skip the steps 4.2.5-4.2.7 below. Instead, wash the cells twice with 1 mL of HBSS with 1% bovine serum albumin (see Supplementary File 1) and resuspend in 1 mL of HBSS with 1% bovine serum albumin. Perform the analysis on the flow cytometer as quickly as possible; otherwise, cell viability may be compromised as the cells are not fixed. - Wash the cells twice with 1 mL of HBSS and resuspend the cells in 900 µL of HBSS.
- Add 100 µL of 37% formaldehyde (CAUTION, see Supplementary Table 2).
- Incubate for 15 min at RT.
- Wash twice with 1 mL of HBSS with 1% bovine serum albumin (BSA), then resuspend the cells in 1 mL of HBSS with 1% BSA.
- Store the samples in a refrigerator at 4 °C. Analyze the cells within 1 month after fixation.
- Analyze the fixed cell suspension by flow cytometry using the appropriate excitation and detection channel.
NOTE: The correct excitation and detection channels may differ based on the instrument being used (see Supplementary Table 3).
- After counting the cells and adjusting the density to 1 x 106 cells per mL with HBSS, transfer 1 mL of this cell suspension to 2 mL microcentrifuge tubes.

Figure 4: Illustrative description of staining and fixation of leukocytes. Please click here to view a larger version of this figure.
5. Flow cytometry
- Acquire data.
NOTE: The procedures to be followed on the flow cytometer may vary depending on the specific cytometer used. In this study, data were acquired using the Attune NTx flow cytometer.- Run the samples.
- Place the sample tube in the sample port.
- Begin acquisition by pressing start and then click on record once the event rate (events/second) stabilizes.
- Record a minimum of 10,000 events for each sample in the singlets gate for reliable analysis.
- Save all data for each sample and back it up on external drives or cloud storage.
- Analyze the data.
NOTE: For Flow Cytometry analysis, several software options are available. In this study, data analysis was performed using the FlowJo v10.8.119.- Load data files: Launch the flow cytometry analysis software and upload the acquired .fcs files from the experiment.
- Visualize the data (Figure 5A): Plot forward scatter (FSC-A) (XX) vs. side scatter (SSC-A) (YY) to assess cell size and granularity.
NOTE: This plot is commonly used to identify cell populations and exclude debris based on their scattering properties. - Singlet cell gating and multiplets exclusion (Figure 5B): Use forward scatter area (FSC-A) vs forward scatter height (FSC-H) plot to exclude multiplets. Draw a gate to include all the events aligned in the graph (single cells) and exclude the unaligned (multiplets). If the sample has events with very low FSC, exclude those by not including them on the single cell's gate, remaining only singlet cells.
- Identifying leukocyte populations (Figure 5C): Distinguish the 3 leukocyte populations based on FSC-A/SSC-A profile: FSC-Ahigh/SSC-Ahigh are granulocytes the FSC-Amedium/SSC-Amedium are the monocytes and FSC-Alow/SSC-Alow are the lymphocytes.
- Viability gating (Figure 5D): Set up the threshold for viability dye staining on the corresponding fluorescence channel (NIR, RL3-A). Live vs dead cells are defined based on the staining intensity with LIVE/DEAD Fixable Dead Cell Stain: dim stained cells are defined as the live cells, and the strongly stained cells are defined as dead cells.
- Population statistics: Look at the statistics provided by the software, including total events (cells) in each gate and percentages of total and gated cells.
- Export data: Export the data to a spreadsheet and use appropriate statistical software (GraphPad Prism or R) for further statistical analysis.
NOTE: The choice of analysis methods will depend on the objectives of the research.

Figure 5: Flow cytometry gating strategy for viability assessment in head kidney cells: (A) Represents FSC-A/SSC-A profile of all collected events. (B) Represents multiplets exclusion based on the definition of singlets region based on linearity on the Forward Scatter (FSC-A)/Forward Scatter (FSC-H) graph. (C) Represents the 3 major populations that were defined based on FSC-A/SSC-A, after multiplets exclusion. (D) Represents a histogram showing Live/Dead Viability dye staining. To set up the threshold that allows to distinguish live and dead cells, leukocytes were submitted to mild heat shock (50 ˚C, 7 min) and then stained with viability dye. High-staining intensity cells positive (++) are the dead cells, and low-staining positive cells (+-) are the live cells. Please click here to view a larger version of this figure.
Results
Figure 6 presents representative flow cytometry data showing leukocyte populations isolated from the head kidney of juvenile gilthead seabream (Sparus aurata) and their cell viability using the protocol described in this study. The figure compares two samples: one with high cellular viability, where the fish were exposed to optimal conditions (Figure 6A), and another with low cellular viability, where the cells were exposed to thermal stress (Figure 6B).
In both samples, the present method effectively identified three main leukocyte populations: lymphocytes (LY), monocytes (MO), and granulocytes (GR). These populations were distinguished based on differences in cell size and complexity, as indicated by forward scatter (FSC-A) and side scatter (SSC-A) plots. Forward scatter (FSC-A) indicates relative cell size, while the side scatter (SSC-A) reflects the internal complexity or granularity of the cells, allowing a clear separation of the populations. Under optimal conditions (Figure 6A), the leukocyte populations were distributed as follows: LY at 31.0%, MO at 38.0%, and GR at 31.0%. However, in the sample exposed to thermal stress (Figure 6B), a shift in the distribution of these populations was observed, with LY decreasing to 21.3%, MO increasing to 45.6%, and GR remaining relatively stable at 33.1%.
Further analysis of cell viability, detailed in Figure 6A1-A3 and Figure 6B1-B3, reveals a significant contrast between the two samples. As can be observed, the sample exposed to optimal conditions (Figure 6A) exhibited higher viability, with a predominance of live cells (represented in blue) across all leukocyte populations. In contrast, the thermally stressed sample (Figure 6B) showed a significant increase in cell death (more intense staining shown in red), with LY showing 50.7% cell death, MO 83.7%, and GR 84.5%.
Overall, the results clearly demonstrate the protocol's effectiveness in both isolating and analyzing leukocyte populations and assessing cell viability. Moreover, the data provide valuable insights into how stressful conditions, such as thermal exposure, influence leukocyte population dynamics and cell viability. The observed shifts in cell distribution and viability under stress suggest that thermal exposure not only disrupts the balance of leukocyte populations but also compromises cell health. These changes may indicate an immune response to stress, pointing to an active defense mechanism triggered by environmental stressors.
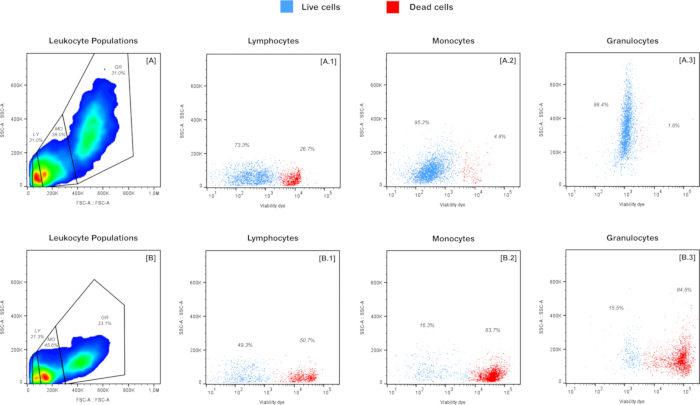
Figure 6: Representative flow cytometry plots illustrating the identification and viability of different leukocyte populations from the head kidney of gilthead seabream (Sparus aurata): (A) Sample with high cell viability, exposed to optimal conditions: The left panel shows the gating of leukocyte populations based on FSC-A/SSC-A, identifying lymphocytes (LY), monocytes (MO), and granulocytes (GR); Viability of LY (A.1), MO (A.2), and GR (A.3). (B) Sample with low cell viability, subjected to thermal stress: The left panel shows the gating of leukocyte populations based on FSC-A/SSC-A, identifying lymphocytes (LY), monocytes (MO), and granulocytes (GR); Viability of LY (B.1), MO (B.2), and GR (B.3). Please click here to view a larger version of this figure.
Supplementary Figure 1: Representative image of a quarantine tank with a total capacity of 660 L. Please click here to download this figure.
Supplementary Figure 2: Image of a RAS system setup. (1) Profilux; (2) Luminaires; (3) Aquariums/Tanks; (4) Refrigerator; (5) Water inlet taps in aquariums; (6) Blue siphon for aquarium water outlets; (7) Sump; (8) Protein skimmer; (9) Mechanical filter (filter bag); (10) Mechanical filter (blue sponge + glass wool); (11) Biological filter (bioballs); (12) Thermostat; (13) Main pump; (14) UV sterilizer; (15) Temperature Sensor; (A) Mechanical filter (blue sponge); (B) Mechanical filter (glass wool). Please click here to download this figure.
Supplementary Table 1:Ingredient composition (%) and proximate analysis (%DM) of the experimental diet used in the study. Please click here to download this table.
Supplementary Table 2: Reagents used in the protocol: Health hazards, hazard statements, precautionary statements, and first aid measures. Please click here to download this table.
Supplementary Table 3: Appropriate excitation and detection channels to use with the amine-reactive LIVE/DEAD fixable dead cell stains. Please click here to download this table.
Supplementary File 1: Calculations for the preparation of solutions. Please click here to download this file.
Discussion
The method developed in this study represents a significant advancement in fish immunology research and promises to improve the understanding of fish immune responses and the sustainability of marine resources. S. aurata is a valuable marine fish species of the family Sparidae serving as an ideal model organism for several reasons, including its ecological and economic relevance, as well as its versatility in laboratory research across several fields of study such as physiology, immunology, toxicology, and aquaculture20. One of the main advantages of S. aurata is its ease of handling and rearing in the laboratory, with well-established husbandry protocols that ensure consistent and reproducible experimental conditions21. Furthermore, its environmental adaptability (i.e., high tolerance to a wide range of environmental conditions, such as temperature and salinity fluctuations) makes it an ideal candidate for studies aimed at improving farming techniques and assessing environmental impacts on marine organisms22. S. aurata, with its well-characterized immune system and availability of genomic data, allows for the unlocking of fish immune responses, pathogen resistance, vaccine efficacy, and overall health20. Given its importance in aquaculture, as well as in the dietary habits of Southern European populations, research on this species can directly impact farming practices, enhancing its production and sustainability while addressing environmental challenges within the industry20.
In this sense, this protocol offers several advantages and potential applications in various research areas, such as: (i) Fish immunology: Provides a precise and detailed analysis of immune cells from the head kidney of S. aurata, allowing the identification and characterization of different leukocyte populations, as well as robust assessments of their cell viability by flow cytometry; (ii) Aquaculture research: Essential to the study of farming techniques, nutrition and disease management, supporting future research aimed at reducing reliance on antibiotics and chemical treatments, thereby promoting more sustainable aquaculture practices; (iii) Ecotoxicology: Valuable in the assessment of the effects prompted by pollutants and other environmental stressors on fish immune health, providing data for environmental risk assessment and the formulation of protective regulations for aquatic ecosystems; (iv) Comparative physiology: Allows researchers to follow up on evolutionary adaptations of immune systems to various environmental challenges, providing a deeper understanding of the genetic and physiological basis of immune function; (v) Marine biology and ecology: Improve understanding of the ecological roles and interactions of S. aurata, providing insights into species distribution, habitat preferences, and the impact of environmental changes on marine biodiversity; (vi) Biomedical research: Fishes are increasingly often used as model organisms in biomedical research. Immune cell isolation and analysis can help understand cell and molecular aspects critical for drug discovery and even medicine.
For many years, the quantification and identification of leukocytes in fish relied on traditional hematological techniques, such as manual counting with Bürker, Neubauer, or Thoma hemocytometers, as well as stained blood smears10,12. Cell viability was also assessed using the trypan blue exclusion test, followed by microscopy counting15,22,23,24,25,26,27,28. While these methods have contributed to our understanding of fish immunology, they have several limitations. Manual leukocyte counting was both time-consuming and labor-intensive, and the accuracy of these techniques was often compromised by operator variability and human error, significantly affecting the consistency of results10.
In 1994, Esteban et al. revolutionized fish leukocyte analysis by introducing a technique to assess the phagocytic defense mechanism in seabass (Dicentrarchus labrax)23. In their study, leukocytes were isolated from peripheral blood, head kidney, and peritoneal exudates using Percoll density gradient centrifugation23. This methodological advancement marked a significant leap forward, enhancing the accuracy and reliability of leukocyte analysis. Following Esteban et al.'s pioneering work, subsequent studies have aimed to refine and optimize flow cytometry techniques for leukocyte analysis, focusing on various aspects such as cell population profiling6,15,16,28,29, cell viability3,6,27,28, and phagocytic activity6,23,24,27,28. Advances in isolation procedures, including the use of different gradient media (e.g., Ficoll vs. Percoll) and optimized centrifugation protocols, have improved the purity and yield of isolated leukocytes1,8,14,26. The development of advanced staining techniques and the use of fluorescent dyes, such as propidium iodide (PI) and 3,3'-Dihexyloxacarbocyanine iodide (DiOC6(3)) have enabled more accurate discrimination between viable and non-viable cells, as well as detailed characterization of leukocyte populations, respectively16,30. One of the major advantages of this technique is its ability to simultaneously distinguish between different leukocyte populations (lymphocytes, monocytes, and granulocytes) and to assess the cell viability of each population. This allows researchers to identify which populations are most affected under specific conditions, providing deeper insights into the immune response and revealing the most vulnerable leukocyte subsets. However, despite these improvements, current methods still face limitations. Most research has focused on adult fish, leaving a gap in the study of juvenile stages. Juvenile fish present unique challenges for leukocyte extraction due to their smaller size and the difficulty in obtaining high-quality samples from their proportionally smaller immune organs17. The study described here specifically addresses these issues by optimizing the protocol for juvenile fish, adjusting the cell suspension ratio, and Percoll solution to improve the quality of leukocyte extraction. Additionally, existing methods are often limited by the number of samples or replicates that can be processed at one time due to the immediate need for leukocyte viability assessment. Any delay in sample handling can adversely affect cell viability, complicating the sampling process and potentially compromising results. To overcome this limitation, this study introduces the use of a novel staining technique that allows for cell fixation and analysis up to 1-month post-fixation. This innovation provides greater flexibility in sampling schedules, allowing researchers to process and analyze samples at their convenience, significantly improving the workflow and reliability of flow cytometry assessments.
The success of this protocol relies on several critical steps, each requiring careful execution to ensure the integrity and viability of the leukocytes, which are subsequently analyzed by flow cytometry. The correct preparation of the euthanasia solution and pH adjustment are critical for effective euthanasia. Improperly prepared solutions may result in incomplete euthanasia or additional stress to the fish18. Accurate and careful dissection of the head kidney is vital to avoid contamination. Maintaining sterility and tissue integrity is crucial to obtain high-quality leukocyte samples. The density, osmolarity, and pH of the Percoll solution must be correctly adjusted for efficient leukocyte separation. Gently aspirating the leukocyte ring with a sterile pipette is critical to avoid contamination and disturbance of the gradient layers. Effective washing of cells post-collection is essential to remove Percoll residues and other impurities. Repeating the washing process ensures a clean cell pellet ready for fixation. The reactive dye should be prepared immediately before use to ensure its effectiveness, as improper handling can compromise its stability. Proper dye addition and incubation time are critical for accurately distinguishing between live and dead cells. Fixing cells with 3.7% formaldehyde is a critical step to preserve cell morphology and ensure accurate identification of dead cells. Formaldehyde concentration must be precise, and incubation times must be strictly followed to avoid inaccurate results. Washing the cells after fixation is important to remove any excess dye and formaldehyde, which could interfere with subsequent analysis. Proper storage of fixed samples and their timely analysis are necessary to maintain data accuracy. Adjusting the excitation and detection channels to match the specific dye used is essential for accurate flow cytometry results.
Despite its numerous advantages, this method has some limitations that must be considered. One key limitation is its species-specific nature, as the protocol is optimized for juvenile gilthead seabream (S. aurata), a marine fish. This means that its effectiveness may vary when applied to other fish species, particularly freshwater fish. Differences in osmotic balance between marine and freshwater species may require adjustments to the buffers used in the protocol, as the solutions suitable for saline conditions may not be appropriate for freshwater species. Additionally, differences in tissue structure, cell density, and physiological responses may require adjustments to the protocol, potentially affecting reproducibility and consistency across different species.
Another important factor is the fish maintenance prior to sample collection. Each species has specific optimal water conditions crucial for maintaining fish health and ensuring the quality of experimental results20. Variations in parameters such as temperature, salinity, and pH can affect cell viability and data quality, as suboptimal conditions may induce physiological stress that compromises cell integrity. To ensure consistent experimental results, it is important to keep all replicates under the same controlled conditions, whether optimal or intentionally suboptimal (depending on the objectives of the study).
Variability in handling and dissection techniques can also impact the quality and quantity of head kidney leukocytes, making the skill and experience of the operator a critical factor for the success of the protocol. Additionally, maintaining cell viability throughout the isolation process can be a challenge, as any delays between euthanasia and cell fixation can result in decreased viability and biased results. Immediate processing of tissues and cells is essential for maintaining high cell viability, but this requirement can be difficult to achieve consistently, especially when working with a high number of samples.
Disclosures
The authors declare no financial, personal, or professional conflicts of interest that could have influenced the research, analysis, data interpretation, writing, or the decision to submit the manuscript for publication.
Acknowledgements
This work was supported by Fundação Portuguesa para a Ciência e Tecnologia (FCT I.P.), under the framework of the project Aqua-CLIMADAPT (PTDC/CTA-AMB/0592/2021, https://doi-org.bdigitaluss.remotexs.co/10.54499/PTDC/CTA-AMB/0592/2021). We acknowledge the BioLab supported by the Applied Molecular Biosciences Unit (UCIBIO) and the Associated Laboratory for Green Chemistry Research Unit – LAQV, which are financed by national funds from FCT/MCTES (UIDB/04378/2020 and UIDB/50006/2020, respectively), as well as the Associate Laboratory Institute for Health and Bioeconomy – i4HB (LA/P/0140/2020). This work was also supported by the European Commission through the GLYCOTwinning project (Grant Agreement No. 101079417) and FCT through InnoGlyco (2022.04607.PTDC). Isa Marmelo also acknowledges FCT I.P. for the PhD grant (2020.04413.BD, https://doi-org.bdigitaluss.remotexs.co/10.54499/2020.04413.BD).
Materials
| Name | Company | Catalog Number | Comments |
| Air Stones | N/A | N/A | |
| Aquafeed | SPAROS, Lda., Portugal | N/A | High-quality diet |
| Automatic Cell Counting Equipment | NanoEnteK, Korea | N/A | EVE automatic cell counter (NanoEnteK) |
| Automatic Water Refrigeration Systems | Foshan Weinuo Refrigeration Equipment Co., Ltd, China | N/A | |
| Bio balls 1.5" Aquarium Pond Filter | TMC Iberia, Portugal | N/A | |
| Bovine Serum Albumin (BSA) | Sigma-Aldrich, Germany | A7906 | |
| Bucket (3 L) | N/A | N/A | To prepare and carry out euthanasia |
| Buckets (5 L) | N/A | N/A | To transport the animals |
| Cell Strainers | Jetbiofil, China | CSS-013-100 | Cell Strainer, 100 μm nylon mesh, Sterile, Yellow |
| Centrifugue | Fisher Scientific, Germany | N/A | accuSpin Micro 17 R |
| Colorimetric Test Kit for Ammonia (NH4+/NH3) | Tropic Marin, USA | N/A | |
| Colorimetric Test Kit for Nitrate (NO3-) | Tropic Marin, USA | N/A | |
| Colorimetric Test Kit for Nitrite (NO2-) | Tropic Marin, USA | N/A | |
| Computer | N/A | N/A | To acquire and analyse the data obtained from the flow cytometer |
| Computerized Control System (Profilux) | GHL, Germany | N/A | ProfiLux 3 Outdoor |
| Deionized water | N/A | N/A | To clean the Flow Cytometer |
| Density Gradient Medium: Percoll | Cytiva, Sigma-Aldrich, Germany | 17-0891-01 | |
| Digital scale | KERN & Sohn GmbH, Germany | N/A | KERN EMS 300-3 |
| Ethanol 70% | Millipore, Supelco, Portugal | EX0281 | To keep the workspace clean |
| EVE Cell Counting Slides | NanoEnteK, Korea | N/A | |
| Falcon Tubes (15 mL) | pluriSelect Life Science, Germany | 05-00002-01 | Sterile |
| Filter bag | TMC Iberia, Portugal | N/A | 400 micron |
| Filter Sponge | N/A | N/A | |
| Flow Cytometer | ThermoFisher Scientific, USA | N/A | Attune flow cytometer |
| FlowJo v10.8.1 Software | BD Life Sciences | N/A | |
| Formaldehyde 37% | Sigma-Aldrich, Germany | 8.18708 | |
| Glass Wool | N/A | N/A | |
| Hanks' Balanced Salt Solution | Merck Life Science S.L, Portugal | H6648 | Modified, with sodium bicarbonate, without phenol red, calcium chloride and magnesium sulfate, liquid, sterile-filtered, suitable for cell culture |
| LIVE/DEAD Fixable Dead Cell Stain Kits | Life Technologies Europe, Netherlands | L10119 | Near-IR fluorescent reactive dye + DMSO |
| Main Water Pumps | EHEIM, Germany | Universal 1200 | |
| Microcentrifuge Tubes (2 mL) | BRAND, Merck, Germany | Z628034 | Sterile |
| Micropipette Tips | Sartorius, Germany | 790010, 790200, 791000 | Compatible with Sartorius, 1-10 µL, 2-20 µL, 20-200 µL, 100-1000 µL |
| Micropipettes | Sartorius, Germany | 728020, 728030, 728060, 728070 | Sartorius ProlinePlus 1-10 µL, 2-20 µL, 20-200 µL, 100-1000 µL |
| Mini Cell Strainers | pluriSelect Life Science Global Headoffice, Germany | 43-10100-50 | PluriStrainer 100 µm nylon mesh, Sterile |
| Multi-Parameter Measuring Instrument | WTW, Germany | Multi 3420 SET G + IDS digital conductivity cell (TetraCon 925) + Optical IDS DO sensor (FDO 925) + IDS pH-electrode (SenTix 940) | |
| Pasteur Pipettes (1 mL, 5 mL) | Humeau Expert du laboratoire, France | 248295 | Sterile |
| Petri dishes | Sarstedt, Germany | 82.1194.500 | 60 x 15 mm, Polystyrene, Sterile |
| pH Meter | Hanna Instruments Inc., Romania | HANNA HI2211 | |
| Polystyrene round-bottom Falcon tubes (5 mL) | Fisher Scientific, Germany | 14-959-2A | Sterile |
| Portable Precision Thermometer | Ebro Electronic, Germany | N/A | TFX 430 |
| Protein Skimmers | Mantis | N/A | Tornado 120 |
| Quality control beads/Performance test beads | Thermofisher Scientific, USA | N/A | |
| Quarantine Tanks | N/A | N/A | Tanks with 660 L total volume |
| Rectangular Glass Tanks/Aquariums | N/A | N/A | Tanks with 200 L total volume |
| Refrigerator | N/A | N/A | To store the samples at 4 °C |
| Ruler 30 cm | N/A | N/A | To measure the fish's length |
| Sodium bicarbonate | Honeywell Fluka, Germany | 31437 | Sodium hydrogen carbonate (NaHCO3) |
| Sodium chloride | Sigma-Aldrich, Germany | S9888 | |
| Sterile Dissection Tools | N/A | N/A | (e.g. scalpel, scissors, fine-tipped forceps, dissecting tray/board) |
| Submerged Digital Heaters | TMC Iberia, Portugal | 300 W, V2Therm Digital Heaters | |
| Syringes 1 mL | IVFSTORE, USA | 8300014579-MEA | Sterile, HSW Soft-Ject Syringes to macerate head-kidney |
| Temperature Sensors | GHL, Germany | PT 1000 | |
| Tricaine (MS-222) | ThermoFisher Scientific, Germany | 118000500 | Ethyl 3-aminobenzoate, methanesulfonic acid salt, 98% (C10H15NO5S) |
| Ultraviolet Water Sterilizer | EHEIM, Germany | 5305010 | ClearUVC-36 |
| Water Bath | Fisher Scientific, Germany | N/A | Fisherbrand IsotempTM (P/N U01318) |
| Water-resistant Luminaires | N/A | N/A |
References
- Samaï, H.C. et al. Procedures for leukocytes isolation from lymphoid tissues and consequences on immune endpoints used to evaluate fish immune status: A case study on roach (Rutilus rutilus). Fish Shellfish Immunol. 74, 190-204 (2018).
- Mokhtar, D. M., Zaccone, G., Alesci, A., Kuciel, M., Hussein, M. T., Sayed, R. K. A. Main components of fish immunity: An overview of the fish immune system. Fishes. 8 (2), 93 (2023).
- Lulijwa, R. et al. Characterisation of Chinook salmon (Oncorhynchus tshawytscha) blood and validation of flow cytometry cell count and viability assay kit. Fish Shellfish Immunol. 88, 179-188 (2019).
- Uribe, C., Folch, H., Enriquez, R., Moran, G. Innate and adaptive immunity in teleost fish: a review. Vet Med. 56 (10), 486-503 (2011).
- Bjørgen, H., Koppang, E. O. Anatomy of teleost fish immune structures and organs. Immunogenetics. 73 (1), 53-63 (2021).
- Santos, R. A. et al. In vitro modulation of gilthead seabream (Sparus aurata L.) leukocytes by Bacillus spp. extracellular molecules upon bacterial challenge. Fish Shellfish Immunol. 121, 285-294 (2022).
- Witeska, M., Kondera, E., Ługowska, K., Bojarski, B. Hematological methods in fish - Not only for beginners. Aquaculture. 547, 737498 (2022).
- Titus, J. et al. Development and validation of a flow cytometry method to examine circulating leukocyte subpopulations in barramundi (Lates calcarifer). Comp Immunol Rep. 6, 200142 (2024).
- Marmelo, I. et al. Eco-innovative aquafeeds biofortified with Asparagopsis taxiformis to improve the resilience of farmed white seabream (Diplodus sargus) to marine heatwave events. Heliyon. 10 (15), e35135 (2024).
- Seibel, H., Baßmann, B., Rebl, A. Blood Will Tell: What Hematological Analyses Can Reveal About Fish Welfare. Front Vet Sci. 8, 616955 (2021).
- Franke, A., Beemelmanns, A., Miest, J. J. Are fish immunocompetent enough to face climate change? Biol Lett. 20 (2), 20230346 (2024).
- Fazio, F. Fish hematology analysis as an important tool of aquaculture: A review. Aquaculture. 500, 237-242 (2019).
- Fazio, F., Saoca, C., Costa, G., Zumbo, A., Piccione, G., Parrino, V. Flow cytometry and automatic blood cell analysis in striped bass Morone saxatilis (Walbaum, 1792): A new hematological approach. Aquaculture. 513, 734398 (2019).
- Lulijwa, R., Alfaro, A. C., Merien, F., Meyer, J., Young, T. Advances in salmonid fish immunology: A review of methods and techniques for lymphoid tissue and peripheral blood leucocyte isolation and application. Fish Shellfish Immunol. 95, 44-80 (2019).
- Esteban, M. Á., Muñoz, J., Meseguer, J. Blood cells of sea bass (Dicentrarchus labrax l.). Flow cytometric and microscopic studies. Anat Rec. 258 (1), 80-89 (2000).
- Inoue, T., Moritomo, T., Tamura, Y., Mamiya, S., Fujino, H., Nakanishi, T. A new method for fish leucocyte counting and partial differentiation by flow cytometry. Fish Shellfish Immunol. 13 (5), 379-390 (2002).
- Ye, R. R. et al. Immune competence assessment in marine medaka (Orzyias melastigma)-a holistic approach for immunotoxicology. Environ Sci Pollut Res. 24 (36), 27687-27701 (2017).
- American Veterinary Medical Association. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. American Veterinary Medical Association, Schaumburg, IL (2020).
- FlowJoTM Software (for Windows). Version v10.8.1 (BD Life Sciences). At < https://www.bdbiosciences.com/en-us/products/software/flowjo-v10-software> (2023).
- Mhalhel, K. et al. Review on Gilthead Seabream (Sparus aurata) aquaculture: Life cycle, growth, aquaculture practices and challenges. J Mar Sci Eng. 11 (10), 2008 (2023).
- Toni, M., Manciocco, A., Angiulli, E., Alleva, E., Cioni, C., Malavasi, S. Review: Assessing fish welfare in research and aquaculture, with a focus on European directives. Animal. 13 (1), 161-170 (2018).
- Kır, M. Thermal tolerance and standard metabolic rate of juvenile gilthead seabream (Sparus aurata) acclimated to four temperatures. J Therm Biol. 93, 102739 (2020).
- Esteban, M. Á, Meseguer, J. Phagocytic defence mechanism in sea bass (Dicentrarchus labrax L.): An ultrastructural study. Anat Rec. 240 (4), 589-597 (1994).
- Esteban, M. Á., Mulero, V., Muñoz, J., Meseguer, J. Methodological aspects of assessing phagocytosis of Vibrio anguillarum by leucocytes of gilthead seabream (Sparus aurata L.) by flow cytometry and electron microscopy. Cell Tissue Res. 293 (1), 133-141 (1998).
- Rodríguez, A., Esteban, M. Á., Meseguer, J. Phagocytosis and peroxidase release by seabream (Sparus aurata L.) leucocytes in response to yeast cells. Anat Rec A Discov Mol Cell Evol Biol. 272 (1), 415-423 (2002).
- Hamoutene, D., Payne, J. F., Volkoff, H. Effects of tebufenozide on some aspects of lake trout (Salvelinus namaycush) immune response. Ecotoxicol Environ Saf. 69 (2), 173-179 (2007).
- Pierrard, M.-A., Roland, K., Kestemont, P., Dieu, M., Raes, M., Silvestre, F. Fish peripheral blood mononuclear cells preparation for future monitoring applications. Anal Biochem. 426 (2), 153-165 (2012).
- Guardiola, F. A., Logothetis, P., Meseguer, J., Esteban, M. Á. Evaluation of silver nanospheres on viability and innate cellular parameters of gilthead seabream (Sparus aurata L.) head-kidney leucocytes. Fish Shellfish Immunol. 69, 99-107 (2017).
- Campos-Sánchez, J. C., Guardiola, F. A., Esteban, M. Á. In vitro effects of cantharidin on gilthead seabream (Sparus aurata) head-kidney leucocytes. Fish Shellfish Immunol. 123, 20-35 (2022).
- Korytář, T., Dang Thi, H., Takizawa, F., Köllner, B. A multicolour flow cytometry identifying defined leukocyte subsets of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 35 (6), 2017-2019 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved