Case Report
Laparoscopic S7 Hepatectomy with Positive Fluorescence Staining
In This Article
Summary
The protocol demonstrates that, under laparoscopic ultrasound guidance, the S7 segment of the liver can be successfully stained by puncturing the visceral and diaphragmatic branches of the tumor, facilitating an anatomical hepatectomy of the S7 segment.
Abstract
Laparoscopic liver resection for tumors located in the S7 segment of the liver typically adopts a traditional surgical approach. The main goal of this procedure is to accurately dissect the liver pedicle of the S7 segment. Dissecting the hepatic pedicle of the S7 segment along the hepatic hilum requires a relatively long path within the liver, which increases the risk of losing orientation and potentially injuring the adjacent hepatic pedicle of the S5 and S6 segments, thereby compromising the liver resection plane. A positive staining method was used to directly puncture the corresponding portal vein under ultrasound guidance (commonly for S7 and S8 segments) to specifically color the target liver segment, thereby avoiding extensive resection of the liver parenchyma, and reducing damage to the surrounding healthy liver tissue. However, the positive staining method requires a specific foundation in intraoperative procedures, which can be challenging for surgeons and has a certain learning curve. Currently, technologies such as three-dimensional reconstruction territory analysis, intraoperative ultrasound, and indocyanine green fluorescence imaging are popular and commonly used in laparoscopic liver resection. In this protocol, under laparoscopic ultrasound guidance, the tumor basin was punctured through the visceral and diaphragmatic surfaces of the liver to stain segment S7. Laparoscopic resection of segment S7 within the portal territory anatomic liver was successfully performed, further confirming the feasibility and advantages of positive fluorescence staining in laparoscopic liver resection at this stage.
Introduction
Primary liver cancer is currently the fourth most common malignant tumor and the second leading cause of cancer-related death in China, posing a serious threat to the lives and health of people globally1. For hepatocellular carcinoma (HCC), surgical resection has long been the primary treatment option. With the advancements in minimally invasive technology, there has been a growing number of reports on laparoscopic anatomical liver resection for treating HCC. For laparoscopic hepatectomy located in various liver segments, including specialized segments (I, IVb, VII, and VIII), relevant studies have shown that this method is safe and effective2.
The concept of anatomical liver resection was first proposed by Makuuchi et al. in 19853,4. The correct procedure is to segment according to the portal vein territory staining and perform complete resection of the portal vein territory staining to which the tumor belongs5. As HCC mainly spreads along the portal vein, in theory, this approach can provide better oncological efficacy and achieve true anatomical liver resection for tumors in different locations6. However, in the past, due to limitations in technology and equipment, this treatment was uncommon. Most centers performed anatomical liver resection based on the Couinaud liver segmentation method. When the tumor spans multiple liver segments, performing anatomical liver resection can result in excessive removal of healthy liver tissue, thereby increasing surgical risk and postoperative complications. Additionally, potential micrometastatic lesions may persist due to incomplete resection of the tumor portal vein territory7,8.
With the advancements in technology and equipment, we can define the tumor portal vein territory based on preoperative three-dimensional reconstruction. This helps clinicians determine the resection range, perform ultrasound-guided puncture and staining of the portal vein territory staining branches to which the tumor belongs during surgery, and determine the liver section plane using indocyanine green fluorescence imaging to achieve more accurate anatomical liver resection9. However, for laparoscopic anatomical liver resection of liver segment S7, the operation is more challenging because the liver pedicle is deeply hidden in the liver parenchyma and localized proximally to the dorsal and cephalad sides, thus resulting in a longer operation time and greater trauma10. The positive staining method involves direct puncture of the corresponding portal vein under ultrasound guidance (typically used in liver segments S7 and S8) so that the target liver segment is directly stained. This helps the surgeon avoid cutting an excessive amount of liver parenchyma, thereby maximizing the protection of functional liver volume11. However, the positive staining method requires a specific intraoperative ultrasound foundation, the correct identification of intrahepatic ducts, and the appropriate use of intraoperative portal vein puncture techniques. It also places a high demand on the surgeon and is associated with a prerequisite learning curve.
In the patient described here, the tumor was located in segment S7 of the liver. The preoperative three-dimensional reconstruction revealed two portal vein branches. Because the S7 trunk is short and close to the root of the segment S6 portal vein, it was punctured along the diaphragmatic and visceral surfaces of the liver. Indocyanine green was injected to stain the target liver segment, and the liver was resected following the fluorescent signal that guided the procedure and ensured smooth operation.
The aim of the liver S7 segment resection method demonstrated here is to further promote the concept of portal territory staining-guided anatomic liver resection and highlight the advantages of S7 segment-positive staining resection. This procedure minimizes the volume of healthy liver tissue removed during tumor resection while maximizing tumor removal efficiency.
CASE PRESENTATION:
A 30-year-old male was admitted to Foshan Fosun Chancheng Hospital on 2023-02-02. The patient was found to have a space-occupying lesion in his liver at another hospital 1 month prior, without discomfort. He otherwise had a history of good health.
Diagnosis, Assessment, and Plan:
Diagnosis: Hepatocellular carcinoma.
Assessment: ALT (alanine aminotransferase): 123 U/L, AST (aspartate aminotransferase): 34 U/L, hemoglobin: 141 g/L, platelet count: 125 x 109 cells/L, albumin: 40.5 g/L, total bilirubin: 10.1 µmol/L, creatinine: 67 µmol/L, prothrombin time (PT): 14.1 s, hepatitis B surface antigen positive, HBV DNA(Hepatitis B virus DNA): 3.51 x 106 IU/L, abnormal prothrombin (PIVKA-II): 21 mAU/mL, AFP (alpha-fetoprotein): 56.29 µg/L, CA199 (carbohydrate antigen199): <0.8 U/mL, CEA (carcinoembryonic antigen): 4.65 U/mL, cholinesterase: 7128 U/L, Child-Pugh grade A. Enhanced CT (computerized tomography scan) and enhanced (gadoxetate disodium) MRI (magnetic resonance imaging) of the upper abdomen: 1 cm mass in the liver S7 segment, three-dimensional reconstruction territory analysis (see Figure 1). The remaining liver volume was 78.8%.

Figure 1: Three-dimensional reconstruction analysis. The location of the tumor, three-dimensional reconstruction of the tumor-related portal and hepatic veins, and important blood vessels near the tumor. Abbreviations: v7 = segments of 7 branches of a hepatic vein; PPC = posterior portal vein C; PPD = posterior portal vein D; IHV = inter territory hepatic vein; RHV = right hepatic vein. Please click here to view a larger version of this figure.
Plan: Laparoscopic S7 hepatectomy with fluorescence-positive staining was planned. Step 1: CT and three-dimensional reconstruction were used for territory analysis. The tumor was located in the posterior portal vein C (PPc) and posterior portal vein D (PPd) territories of the S7 segment of the liver (referring to the right posterior portal vein classification of Japanese scholars4; refer Figure 2). Step 2: Intraoperative ultrasound showed that the portal vein to which the tumor belonged had two vascular branches. Step 3: Target liver pedicles PPc and PPd were severed, and the veins between liver regions S6 and S7 and the right hepatic vein were fully exposed under fluorescence guidance. Step 4: Tumor resection guided by fluorescent staining.

Figure 2. Preoperative CT scan. (A) CT section of tumor-associated portal vein of PPd (red arrow). (B) CT section of tumor-associated portal vein of PPc. Please click here to view a larger version of this figure.
Protocol
This protocol followed the guidelines of the Human Research Ethics Committee of the Foshan Fosun Chancheng Hospital. Written informed consent was obtained from patient for participation in this study.
1. Preoperative preparation
- Patient preparation: The patient was placed in a supine position, with the head elevated and feet lowered and tilted to the left by approximately 30°. General anesthesia was administered, including tracheal intubation. Abdominal disinfection and draping of the surgical area were performed.
- Trocar layout: A 1.2 cm trocar (observation hole) was inserted after cutting the skin horizontally 1 cm to the right of the navel with a surgical knife. Then, a 1.0 cm trocar was inserted at the intersection of the midclavicular line, 5 cm below the right costal margin, a 0.5 cm trocar was inserted below the right costal margin and the axilla, a 1.2 cm trocar was inserted below the xiphoid process, and a 0.5 cm trocar 3 cm to the left of the midpoint of the line connecting the umbilicus and the xiphoid process. The surgeon stands on the right, and the assistant on the left of the patient. The camera was placed in the observation hole.
- Abdominal exploration: Intraoperative ultrasound scans were performed along the portal and hepatic veins to determine the relationship between the tumor and the duct, confirmed by three-dimensional reconstruction. Laparoscopic exploration of the liver and abdominal cavity revealed no other lesions or metastases. Ultrasound localization of the anterior portal (AP) and PP revealed that the PP was type B.
NOTE: Intraoperative ultrasound evaluates the tumor size, location, intrahepatic metastasis, and its relationship with the surrounding blood vessels.
2. Surgical procedure
- Separation of the perihepatic ligament: An ultrasonic knife was used to cut off the round and falciform ligaments of the liver. The second hepatic portal was dissected, exposing the root of the right hepatic vein. The right coronary ligament and the right triangular ligament were cut. A stitch was used to ligate the three short hepatic veins dorsally to the right side of the inferior vena cava to completely free the right liver.
- Occlusion band: An ultrasonic knife was used to free the adhesions around the gallbladder to expose the foramen of the Venturi. Stomach gastric forceps were used along the foramen of Venturi and an occlusion band was placed in the first hepatic portal.
- Puncture and resection
- PPc was visible through the right main operation port on the diaphragmatic surface, and PPd was visible on the visceral surface. The PPc and PPd were punctured using intraoperative ultrasonography guidance with a probe and a puncture hole.
- Diaphragmatic surface puncture (Figure 3 and Figure 4): The probe was inserted into the main operative hole under the right costal margin. The PPC was visible on the diaphragmatic surface, and the long diameter of the PPC was exposed. The puncture point from the root of the PPC was selected. A 21G percutaneous transhepatic cholangial (PTC) needle was used to prick the bile duct. The one face, three points, and four horizontal fingers method was used.
- On one side, the midpoint of the left and right adjustment rods was used for intraoperative ultrasound as the aiming point, and the probe rod was used as the vertical plane for in-plane puncture. The three points selected were the skin puncture point, the intraoperative probe puncture hole, and the target liver pedicle puncture point. The length of the four horizontal fingers was used to measure the skin puncture point approximately at the intersection of the vertical plane of the probe rod and the skin in front of the trocar.
- The PTC needle was held so the bevel faces the ventral distal side. The needle core was removed, and 3 mL of 0.025 mg/mL indocyanine green was slowly injected. The diaphragmatic surface was visualized using fluorescence imaging.
- Visceral surface puncture (Figure 5 and Figure 6): The puncture hole location was selected under the xiphoid process for probe insertion. PPd was selected as the puncture point, and 3 mL of 0.025 mg/mL indocyanine green dilution was slowly injected.
- At this time, the visceral surface of the liver S7 segment was fluorescently imaged and used to determine the resection margin. An elastic traction rope was used to pull the liver S7 segment from the lower edge of the liver S6 segment to the left lower abdomen.
- The liver tissue was cut from the caudal to the cephalic side and along the boundary between the fluorescent and non-fluorescent segments, along the interterritory hepatic vein (IVH) between liver segments S6 and S7 and the right hepatic vein (Figure 7).
- Along the right edge of the right hepatic vein, the liver S7 reflux vein was ligated using ligating clips and disconnected from the two branch liver pedicles of liver section S7. An ultrasonic scalpel and bipolar electrocoagulation were used to cut the liver tissue using fluorescent imaging boundaries under anesthesia-assisted low central venous pressure.
- Hemostasis of residual liver: The residual liver was carefully checked, and the bleeding points were closed one by one using bipolar electrocoagulation. Coated Vicryl antibacterial suture was used to stitch close the incision.
- For postoperative pain, intravenous analgesics were administered. During postoperative observation, changes in liver function and bilirubin levels were measured.
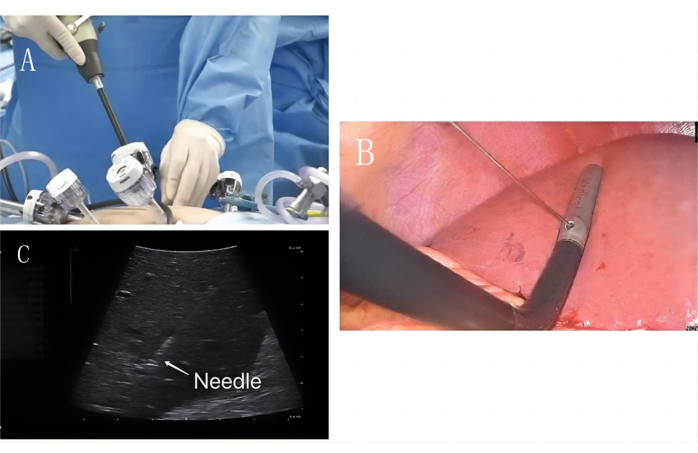
Figure 3: Diaphragmatic surface puncture. (A) Intraoperative ultrasound enters along the main operating hole. (B) Laparoscopic PTC puncture needle is used to puncture along the intraoperative ultrasound fixation hole. (C) PTC puncture needle (white arrow) puncture PPc portal vein image under ultrasound guidance. Please click here to view a larger version of this figure.

Figure 4: Diaphragmatic surface puncture positive staining. PPc portal vein positive staining picture and mark the stained edges with an ultrasonic knife. The green area shows the portal territory to which PPc belongs. Please click here to view a larger version of this figure.
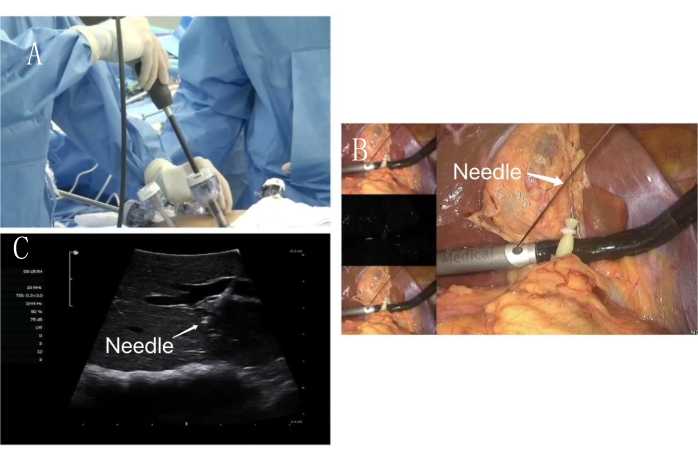
Figure 5: Visceral surface puncture. (A) Intraoperative ultrasound enters along the assistant operation hole. (B) Laparoscopic PTC puncture needle is used to puncture along the intraoperative ultrasound fixation hole. (C) PTC puncture needle (white arrow) puncture PPd portal vein image under ultrasound guidance. Please click here to view a larger version of this figure.

Figure 6: Visceral surface puncture positive staining. PPd portal vein positive staining picture and mark the stained edges with an ultrasonic knife. The green area shows the portal territory to which PPd belongs. Please click here to view a larger version of this figure.
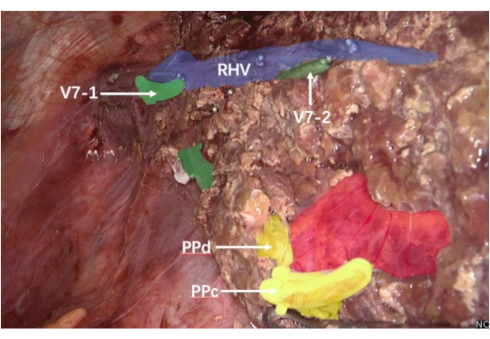
Figure 7: IHV and the right hepatic vein. The purple area shows the right hepatic vein; the green area shows segments of 7 branches of the hepatic vein; the yellow area is a cross-section of the PPC and PPD; the red area is the right posterior hepatic pedicle. Abbreviations: v7-1= The first one of the S7 segment branches of the hepatic vein; v7-2= The second one of S7 segment branches of the hepatic vein; RHV = right hepatic vein; PPc: C branch of the portal vein in the right posterior lobe of the liver; PPd: D branch of the portal vein in the right posterior lobe of the liver. Please click here to view a larger version of this figure.
Results
In this case report, laparoscopic portal territory staining-guided anatomical liver resection of the S7 segment was successfully performed, with positive staining along the diaphragmatic and visceral surfaces (Figure 6 and Figure 7). Resection of a single 1 cm tumor was done. The operative time was 210 min, and intraoperative blood loss was 100 mL. The postoperative hospitalization duration was 7 days, and the patient is still under continuous follow-up. Follow-up is performed every 2 months for 2 years, including liver function tests, tumor marker testing, and imaging-based examinations (ultrasound, CT, or MRI). The post-operative MRI is shown in Figure 8.
Specimen examination: The results of the postoperative pathological examination revealed HCC grade III (moderately poorly differentiated) with a negative surgical margin. Immunohistochemistry: AFP (+), CK19 (+), glypican-3 (+), hepatocyte (+), CD10 (-), CD34 (+, capillary transformation), CK7 (-), and Ki-67 (+, approximately 30% in the hotspot area).

Figure 8: Postoperative MRI scan. The MRI scan of the patient postoperatively. Please click here to view a larger version of this figure.
Discussion
Currently, liver surgery is entering an era of minimally invasive surgery. There have been reports of anatomical resection of various liver segments in patients with HCC, and the safety of this operation has been verified2. Its efficacy in oncology has attracted increasing attention, and the anatomical hepatectomy approach proposed by Shindoh et al. involves complete resection based on tumor-related territory staining6. Limited by previous techniques and equipment, only Couinaud liver segmentation can be used to achieve a concept similar to that of anatomical resection.
It is well-established that HCC primarily metastasizes along the portal vein, with most patients developing portal vein tumor thrombi. The 32nd Annual Meeting of the Japanese Society of Hepatobiliary and Pancreatic Surgery and the Expert Consensus Meeting on Precise Anatomy for Minimally Invasive Hepatobiliary and Pancreatic Surgery clarified and unified the definition of anatomical hepatectomy, that is, complete removal of the PT liver parenchyma8. Anatomic hepatic segmentectomy is clearly defined as the complete removal of the territory staining the liver segments dominated by the third-level hepatic pedicle. With the development of three-dimensional reconstruction territory staining analysis and indocyanine green fluorescence imaging, anatomical hepatectomy based on tumor-bearing portal vein territory staining has been achieved9. People have begun to find that the resection scope of anatomical liver resection in the portal vein basin significantly differs from that of classic anatomical liver resection. This deviation might lead to residual micrometastases in the portal vein basin and local recurrence. When the tumor spans multiple liver segments, the resection scope of classic anatomical hepatectomy is significantly larger than that of anatomical hepatectomy in the portal vein basin, making it difficult to achieve precise and minimally invasive resection to meet clinical needs.
Laparoscopic portal territory staining-guided anatomical liver resection utilizes preoperative three-dimensional reconstruction to delineate the personalized, tumor-bearing portal vein basin. The method was combined with an indocyanine green fluorescent imaging system during surgery to ensure precise resection.
Previously, for classic anatomical hepatectomy, Ferrero et al. dissected a small amount of liver tissue from the dorsal side to accurately control the liver pedicle of the S7 segment, searched for the main trunk of the right hepatic vein using the cephalad hepatic vein approach, and followed the main trunk to sever the liver and complete the anatomical liver S7 resection2. Morise et al. performed laparoscopic anatomical S7 liver resection using a thoracic approach9, while GoroHonda et al. resected the S7 segment of the liver using the dorsal caudal approach12. Chen et al. advocated laparoscopic orthotopic hepatectomy using S713; however, for laparoscopic portal territory staining-guided anatomic S7 segment liver resection, if the target liver pedicle is obtained through the classic surgical approach, a large amount of liver parenchyma needs to be cleaved. If staining is performed on this basis, some liver segments that belong to the tumor watershed may not be stained due to the already cut liver tissue. This will affect the staining and tumor removal efficacy.
The key step in laparoscopic portal territory staining-guided anatomic S7 segment liver resection is the precise puncture of the target hepatic pedicle under intraoperative ultrasound guidance. Only by securing the tumor-related territory and staining branches can fluorescence staining be performed to guide the surgery. The laparoscopic portal vein puncture staining method has a steep learning curve, necessitating not only proficiency in understanding liver anatomy but also having a solid foundation for interpreting ultrasound findings during laparoscopy. This method thus presents an internationally acknowledged technical challenge.
Although beginners can quickly learn this technology using the puncture guide holes on some ultrasound equipment because the angle between the puncture tunnel and the probe is fixed at 60°, it requires a precise selection of the puncture point through the abdominal wall; otherwise, puncture failure may occur14. To address this, we propose a one-side, three-point, and four-horizontal-finger method: on the one side, the midpoint of the left and right adjustment rods was used as the aiming point, and the probe rod was used as the vertical plane for in-plane puncture. On the three points, the skin puncture point, intraoperative probe puncture hole, and target liver pedicle puncture point, and on the four horizontal fingers, the skin. The puncture point was located approximately at the intersection of the four horizontal fingers, from the vertical plane of the probe rod and the skin in front of the trocar. The portal vein was punctured from the diaphragmatic and visceral surfaces of the liver to achieve laparoscopic portal territory staining-guided resection of the anatomic S7 segment of the liver and verify the feasibility and safety of the operation.
Throughout the puncture procedure, we highlighted several critical details that require attention, including the type of puncture needle, the surgeon's stance, the angle and direction of the needle tip, and the rate at which the assistant administers the medication. The size of the puncture needle should ideally range from 18G to 21G (0.8 mm-1.2 mm), and the injection flow rate should be carefully controlled. The surgeon's stance generally follows the contralateral principle of the tumor: if the tumor is on the right side of the liver, the surgeon stands on the left side, and vice versa. The needle tip was inserted into the liver at an upward angle and directed towards the distal end of the target liver pedicle to prevent drug reflux and contamination of the other branches during injection. The direction of the needle tip was as follows: one point, three spots, and four horizontal fingers, with the needle entering the liver facing upward. Once the needle tip entered the liver, adjustments were minimized to avoid liver damage, and the direction of the needle tip could become lost owing to probe displacement during the procedure. The probe should then be gently rotated to locate the needle tip rather than adjusting the direction of the needle to align with the ultrasound plane. If the direction of the needle tip is not consistent with the direction of the target liver pedicle, the tip can be withdrawn and combined with the ultrasound, and the needle can be inserted again. Regarding the rate of medication administration, it is crucial to maintain control over the injection speed under direct ultrasound visualization to ensure that the medication follows the direction of the portal vein blood flow.
The failure of laparoscopic portal vein puncture staining is mainly divided into two aspects: too many branches of the tumor that cannot be fully stained and staining failure caused by drug reflux. Regarding these two limitations, if multiple portal vein branches are found to supply blood to the tumor during surgery, but the preoperative 3D reconstruction does not reflect this, partial watershed staining and fluorescence boundaries can be used to cleave the liver parenchyma, locate and block the hepatic pedicle, and then detach the remaining liver parenchyma, according to the ischemic line or reverse staining. If the above details are not taken into account and drug reflux occurs, the fluorescence device can be adjusted to black-and-white mode to improve contrast.
However, compared to the current laparoscopic anatomical liver segments S7 and S815, the portal vein aspiration-positive staining method has the following advantages. Theoretically, the positively stained liver segment of the portal vein watershed is the closest presentation to the real anatomical watershed, which can effectively avoid residual nonfunctional liver volume and reduce the risk of tumor micrometastasis and recurrence16. Accurate positioning of the target liver pedicle without dissecting the hepatic portal and damaging any liver parenchyma can effectively avoid postoperative complications such as bile leakage. Under the premise of successful staining, complete separation of the liver parenchyma along the fluorescent interface effectively prevents damage to the hepatic vein and reduces the risk of bleeding.
Further research on randomized controlled trials of laparoscopic portal territory anatomic liver resection for the treatment of HCC should be conducted to investigate the superiority of this approach for oncology. Additionally, the importance of ultrasound in hepatobiliary surgery is becoming increasingly self-evident.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors have nothing to disclose.
Materials
| Name | Company | Catalog Number | Comments |
| Coated Vicryl Plus Antibacterial Suture | Ethicon, Inc. | 3650118 | The product is suitable for the placement and/or ligation of soft tissues |
| Color Doppler ultrasound diagnostic scanner | BK Medical | 20153251933 | intraoperative ultrasound |
| Disposable laparoscopic puncture device and puncture sheath | Jiangsu Fenghe Medical Equipment Co., Ltd | 20182021588 | Used for laparoscopic examination and surgical procedures, to puncture the abdominal wall tissue of the human body and establish a working channel for abdominal surgery |
| Four way curved electron convex array laparoscopic intraoperative probe | BK Medical | 20153251933 | Used for intraoperative examination and interventional treatment in various laparoscopic surgeries |
| HAKKO SONOGUIDE PTC NEEDLE | Baguang Trading (Shanghai) Co., Ltd | 20172146872 | Percutaneous liver bile duct puncture needle |
| Indocyanine Green for Injection | DANDONG YICHUANG PHARMACEUTICAL | ICP-09018669-1 | Assessment of liver reserve function and liver imaging |
| WECK Hem-o-lok | Teleflex Medical | 20143466018 | Ligation of blood vessels or tissues |
References
- Han, B. F., et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. 4 (1), 47-53 (2024).
- Ishizawa, T., et al. Laparoscopic segmentectomy of the liver: from segment I to VIII. Ann Surg. 256 (6), 959-964 (2012).
- Makuuchi, M., et al. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet. 161 (4), 346-350 (1985).
- Takamoto, T., Makuuchi, M. Precision surgery for primary liver cancer. Cancer Biol Med. 16 (3), 475-485 (2019).
- Cho, A., et al. Relation between hepatic and portal veins in the right paramedian sector: proposal for anatomical reclassification of the liver. World J Surg. 28, 8-12 (2004).
- Shindoh, J., et al. Complete removal of the tumor-bearing portal territory decreases local tumor recurrence and improves disease-specific survival of patients with hepatocellular carcinoma. J Hepatol. 64 (3), 594-600 (2016).
- Shindoh, J., et al. The intersegmental plane of the liver is not always flat - tricks for anatomical liver resection. Ann Surg. 251 (5), 917-922 (2010).
- Ciria, R., et al. A snapshot of the 2020 conception of anatomic liver resections and their applicability on minimally invasive liver surgery. A preparatory survey for the expert consensus meeting on precision anatomy for minimally invasive HBP surgery. J Hepatobiliary Pancreat Sci. 29 (1), 41-50 (2022).
- Zheng, J., et al. Laparoscopic anatomical portal territory hepatectomy with cirrhosis by takasaki's approach and indocyanine green fluorescence navigation (with Video). Ann Surg Oncol. 27 (13), 5179-5180 (2020).
- Kawaguchi, Y., et al. Difficulty of laparoscopic liver resection: proposal for a new classification. Ann Surg. 267 (1), 13-17 (2018).
- Liang, X., et al. Laparoscopic anatomical portal territory hepatectomy using Glissonean pedicle approach (Takasaki approach) with indocyanine green fluorescence negative staining: how I do it. HPB. 23 (9), 1392-1399 (2021).
- Ferrero, A., et al. Laparoscopic right posterior anatomic liver resections with Glissonean pedicle -first and venous craniocaudal approach. Surg Endosc. 35 (1), 449-455 (2021).
- Morise, Z. Laparoscopic liver resection for posterosuperior tumors using caudal approach and postural changes: a new technical approach. World J Gastroenterol. 2016 (47), 10267-10274 (2016).
- Okuda, Y., et al. Intrahepatic Glissonean pedicle approach to segment 7 from the dorsal side during laparoscopic anatomic hepatectomy of the cranial part of the right liver. J Am Coll Surg. 226 (2), e1-e6 (2018).
- Cao, J., et al. Totally laparoscopic anatomic S7 segmentectomy using in situ split along the right intersectoral and intersegmental planes. Surg Endosc. 35 (1), 174-181 (2021).
- Wang, X., Tong, H., Li, J., Wang, H. I. Indocyanine green fluorescence-guided laparoscopic anatomical segmentectomy of liver segment 6: Surgical strategy and technical details. Ann Surg Oncol. 31 (10), 6546-6550 (2024).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved