Method Article
Effects of Different Connection Modes of Electroacupuncture on Electrocardiogram and Nerve Discharge in Rats
In This Article
Summary
Until now, there is no uniform method for electroacupuncture connection in clinical and fundamental research. We present a protocol that attempted to compare the effects of different connection methods on electrocardiogram (ECG) and neural discharge in rats to explore the most appropriate way to connect the electroacupuncture instrument.
Abstract
Electroacupuncture (EA) is one of the most commonly used methods in acupuncture and has a good effect on pain, depression, sensory movement disorders, and other diseases. The effectiveness of EA is influenced by many factors, such as the accuracy of acupoint selection, the duration and course of EA treatment, and EA parameters. However, it has rarely been discussed whether the positive and negative electrodes of the EA instrument with acupoints at different locations and distances have an effect on the curative effect. In this experiment, we observed the effects of connecting the EA instrument to acupoints at different positions and distances on ECG and vague nerve discharge in rats and preliminarily explained whether the electric field formed by different modes of EA connection has an effect on the function of the body. The connection modes of EA in this experiment included the same acupoints on both sides of the body, the same meridian or different meridian acupoints on the same side of the body, and two needles in the same acupoint area. The results showed that when the positive and negative poles were connected to the acupoints on both sides of the body, the recording of ECG and vagal nerve activity was disturbed (the same fore and hind limbs); when the acupoints were connected on the same side of the body, the smaller the distance between the two needles, the smaller the effect on ECG and vagal nerve activity recording, and the effect increased with increasing current; when the acupoints were in the same acupoint area, the recording of ECG and vagal nerve activity was not disturbed if the two needles did not form a short circuit.
Introduction
Electroacupuncture (EA) is a type of acupuncture therapy for the treatment of diseases by applying pulsed current output from an instrument to meridian points of the human body. EA has the advantages of stable and adjustable stimulation parameters, which can be quantified, timed, and labor-saving, and has special advantages in the treatment and scientific research of certain diseases, such as the study of the treatment mechanism for various neuralgia and acupuncture anesthesia, acupuncture analgesia and so on1.
Some factors influence the effectiveness of EA, such as acupoint accuracy, treatment duration, and EA parameters, such as waveform, pulse intensity, and frequency. The commonly used clinical EA is a two-way EA, which causes less tissue damage and interferes less with the normal physiological functions of the human body. It can also prevent and cure diseases. Human tissues are conductors of complex electrolytes composed of water, inorganic salts, and charged colloids2. When EA is applied to the human body, the charged particles in the electric field will move and cause changes in concentration and distribution that affect the function of the human body. Changes in movement, concentration, and particle distribution are the basis of EA therapy1.
Over the past decade, the number of EA studies has increased for a number of diseases1,2,3. Regarding the connection of the positive and negative electrodes of EA, few studies have been conducted to clarify this in detail. During EA, the positive and negative electrodes on the EA instrument should be connected to two acupuncture needles to act as electrical stimulation. At present, the common modes are as follows: two needles on each side of the body are connected to the same acupoints, and different acupoints on the same side of the body are connected1. However, the effectiveness of these connection methods has yet to be proven. Some scientists have estimated that the two needles on each side of the body cannot be connected to the positive and negative poles; otherwise, the current would affect the heart function, but the two different points on the same side of the body can be connected to the positive and negative poles3. Some researchers believe that acupuncture needles connecting the positive and negative electrodes should be used in the single acupoint area to form electrical stimulation in the acupoint to promote meridian sensing2.
Electroacupuncture at various acupoints can activate or inhibit nerve discharge4,5. Many studies explain the stimulating effect of EA in Tsusanli (ST36) on the vagus nerve6,7,8. However, these studies have not elaborated on the connection of EA and have not explained the differences in the effects of different modes of EA connection. Given the gap in this research, this study used electrophysiological techniques to explain the effects of different EA connections on ECG and nerve activity. The results provide further evidence for the correct connection of the EA.
Protocol
This experimental protocol was approved by the Standardized Laboratory Animal Ethics Review of Beijing University of Traditional Chinese Medicine and was conducted in full accordance with the experimental protocol (Ethical review code: BUCM-2023110901-4046).
1. Animals and grouping
- Animals
- Obtain 48 healthy male SPF rats (8-week old) weighing approximately 200-240 g.
NOTE: For this study, the animals were provided by the Vital River Company [Licence number: SYXK (Beijing) 2020-0033]. - House all rats in the Animal Experimental Center of Beijing University of Traditional Chinese Medicine. During the experiment, ensure that the rats have free access to food and water.
- Maintain the feeding environment based on a circadian rhythm shift of 12 h and control the ambient temperature at 23 ± 2 °C.
- Soak all surgical instruments in 75% alcohol and autoclave. Sterilize the animal handling rooms with UV lamps.
- Perform all animal surgeries under anesthesia. Anesthetize the rat with 20% intraperitoneal urethane injection (1 g/kg) and ensure the rat continues breathing spontaneously.
- Maintain the body temperature of the rat at 37 °C with thermostatic plates during surgery.
- Keep the eyes of the rat moist with erythromycin ointment during the experiment.
- Decapitate and kill the rats under anesthesia at the end of the experiment and store the specimens/samples as necessary.
- Obtain 48 healthy male SPF rats (8-week old) weighing approximately 200-240 g.
- Groups
- Group the rats randomly according to the connection modes. Allot 8 rats to each group randomly.
- Control group (no acupuncture): Feed the rat normally without any treatment.
- Group A, single acupoint in the left forelimb group: Connect the positive and negative EA electrodes to the Quchi point and 3 mm from the Quchi point, respectively (see Figure 1A). Locate the Quchi point (LI11) in the lateral anterior depression of the elbow joint and insert the needle perpendicularly to a depth of ~5 mm after positioning.
- Group B, two acupoints in the left forelimb group: Connect the positive and negative EA electrodes to the Quchi (LI11) and Waiguan (TE5) points, respectively (see Figure 1B). Locate the Waiguan (TE5) point outside the lower 1/6-fold point of the forearm in the radial and ulnar sutures. Insert the needle to a depth of 2 mm.
- Group C, the two acupoints in the left fore and hind limb group: Connect the positive and negative EA electrodes to Quchi (LI11) and Tsusanli (ST36) (see Figure 1C). Locate the Tsusanli (ST36) point 5 mm below the fibular head and posterolateral to the knee and insert the needle perpendicularly to a depth of ~1 cm.
- Group D, the same acupoints in both hindlimbs: Connect the positive and negative EA electrodes with bilateral Tsusanli (ST36) (see Figure 1D).
- Group E, the same acupoints on both forelimbs: Connect the positive and negative EA electrodes to bilateral Quchi (see Figure 1E).
2. ECG connection
- Restrain the rats in the supine position after being thoroughly anesthetized.
- Insert 1/2 inch acupuncture needles parallel to the skin of the right forelimb wrist, right hindlimb, and left hindlimb of the rats, avoiding the muscle.
- Connect the needle handles to the recording electrodes: the positive (white) electrode on the right forelimb, the negative (red) electrode on the left hindlimb, and the reference electrode (black) to the right hindlimb of the rat (see Figure 2).
- Use a physiological signal recorder to record the standard limb lead II surface ECG of rats in real time (sampling rate: 1 KHz, filtering range: low-cut filter, 200 Hz; high-cut filter, 0.8 Hz).
3. Separation of the vagus nerve and recording of the vagal discharge signals
- Make a 2-4 cm incision along the midline of the neck between the larynx and the sternum after supine fixation of the anesthetized rats.
- Sever the right vagus nerve trunk by approximately 1-2 cm after locating the right carotid sheath (containing the carotid artery, vagus nerve, and the sympathetic chain) (see Figure 3A).
NOTE: The sympathetic nerve is located lateral to the carotid artery and accompanies the vagus nerve. The cervical sympathetic nerve is thin compared to the vagus nerve. - Connect the nerve trunk with a pair of copper hook electrodes and clamp the reference electrode to the incision to eliminate interference. Use saline to maintain vagus nerve activity throughout the experiment (see Figure 3B).
- Cover the surface wound with mineral oil to maintain insulation between the electrode and the muscle.
- Use a physiological signal recorder to record the nerve discharge of the rats in real time (sampling rate: 5 KHz, filtering range: low-cut filter, 100 Hz; high-cut filter, 1000 Hz).
4. EA intervention
NOTE: Acupuncture of the rats after the ECG and the vagus nerve discharge were stable.
- Connect the two needle handles separately to the needle clips of the EA instrument.
- Set the EA stimulation to a bidirectional square wave with a wave width of 0.3 ms. Set the stimulation intensity to 0.2 mA, the frequency to 10 Hz, and the stimulation time to 20 min.
- Observe the changes in ECG and nerve discharge of rats under different connection modes of EA in real time. Collect the data for 30 s without any stimulation, 30 s before EA, and 30 s after EA using the physiological signal recorder.
- Record the changes in the heart rates, ECG waveforms, and vagal discharges of the rats using recording software.
5. Statistical analysis of data
- Compare the changes in ECG and nerve discharge in rats in different modes of EA connection (before and after EA).
- Express all experimental data as the mean ± standard error.
- Perform the student's t-test when the data passes the normal distribution test. Perform a paired samples t-test to compare the changes before and after EA and consider p < 0.05 as statistically significant.
Results
Effects of different EA connection modes on ECG in rats
In the Control group, the ECG of normal rats was recorded (see Figure 4A). It was found that the baseline conditions of the rats were significantly different. The heart rates of the rats ranged from 258 to 473 bpm (see Supplementary Table 1).
In Group A, the recording data were similar to the Control group. There was no significant change in heart rate and waveform of rats in Group A after EA stimulation (see Figure 4A). The ECG waveform in Group B showed little intervention compared to the Control group and Group A, and the heart rates in this group could also be recorded in real time. The results in other groups showed that the ECG data were disturbed by EA frequency (see Figure 4A), so the ECG could not be recorded in real-time during EA intervention.Therefore, we recorded the data for 60 s before and after the EA intervention.
Heart rates in Group C and Group D increased after EA was turned off and returned to previous levels in a short time, and the waveform was not affected (see Figure 4A). The connection mode in Group E had the greatest impact on the ECG data. Even a low current (0.1 mA) had a large effect on data recording. After 20 min of electrification, heart rates were significantly increased compared to before EA (p < 0.05) (see Figure 4B), and the animals recovered at varying degrees over the next 30 min. In addition, the ECG waveform of the rats showed a high p-wave phenomenon and an elevated ST segment (see Figure 4C), indicating that the heart was abnormal. These changes continued until the end of the experimental observation.
Effects of different EA connection modes on vagal discharge in rats
The results showed that the vagus nerve discharges were in regular clusters, with the number of clusters varying from 31-66 in 30 s (see Supplementary Table 2), and the recording of nerve discharge was the same as that of the ECG. Except for the Control group and Group A, the nerve discharge data in the other groups were affected by the frequency of EA and could not be accurately recorded during the EA intervention (see Figure 5A). Therefore, we recorded the changes in discharge amplitude (peak values) for 30 s before and after EA intervention and calculated the number of cluster discharges in 30 s. In Group B, vagal discharges were slightly affected by the frequency of EA, and the discharge interference increased with increasing currents. However, the discharge changes in 30 s and the discharge amplitude were not apparent. The electric field caused by the connection current in Group C was significant, but the number of bundle discharges and the maximum discharge value were still stable. Then, in Group D, although the discharge frequency decreased after the EA intervention, the data was not significant compared to before EA (p > 0.05); data in Group E showed the most influence, in which the number of bundle discharges in 30 s significantly decreased (p < 0.05) (see Figure 5B). In contrast, the peak discharge values increased (p < 0.05) (see Figure 5C, Supplementary Table 3).
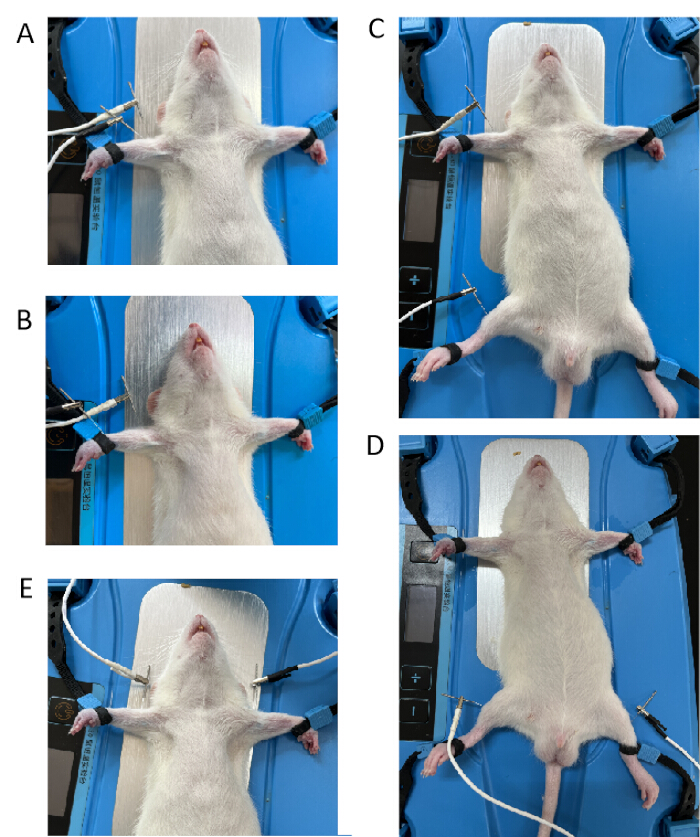
Figure 1: Different connection modes of the positive and negative EA electrodes. (A) The positive and negative EA electrodes were connected to the Quchi point and 3 mm from the Quchi point. (B) The positive and negative EA electrodes were connected to the Quchi (LI11) and Waiguan (TE5) points. (C) The positive and negative EA electrodes were connected to Quchi (LI11) and Tsusanli (ST36) points. (D) The positive and negative EA electrodes were connected to the bilateral Tsusanli (ST36) point. (E) The positive and negative EA electrodes were connected to the bilateral Quchi (LI11) point. Please click here to view a larger version of this figure.

Figure 2: The connection mode of the ECG observation. The needle handles were connected to the positive (white) electrode on the right forelimb and the negative (red) electrode on the left hindlimb, and the reference electrode (black) was connected to the right hindlimb of the rat. Please click here to view a larger version of this figure.

Figure 3: The method of recording the vagal nerve activity. (A) The right vagus nerve was divided. The sympathetic nerve is located lateral to the carotid artery and accompanies the vagus nerve. The left one is the sympathetic nerve, and the right one is the vagus nerve. (B) A pair of copper hook electrodes were used to record the vagal nerve activity, with the reference electrode clamped to the incision. Please click here to view a larger version of this figure.

Figure 4: The recording data of ECG in 6 groups. (A) The real-time recording of ECG with different EA connection modes. The control group showed normal ECG of the rats. The ECG of Group A was the same as that of the Control group. In Group B, the ECG was slightly disturbed after the EA was connected. In Groups C, D, and E, the ECG signal has been completely obscured by the frequency of the EA. (B) In Group E, the heart rate after EA was significantly increased compared to that before EA (p < 0.05). (C) The ECG waveform of the rats showed a high p-wave phenomenon and an elevated ST segment in Group E. Please click here to view a larger version of this figure.

Figure 5: Effects of different EA connection modes on vagal nerve discharge in rats. (A) The real-time recording data of vagal nerve discharge with different EA connection modes. It can be observed that the vagal nerve discharge has the regularity of cluster discharge. The vagal nerve discharge in Group A was similar to that of the Control group, while in Groups B, C, D, and E, the discharge was disturbed by the frequency of the EA instrument. (B) The data in Group E showed the greatest influence, with a significant decrease in the number of cluster discharges in 30 s (p < 0.05). (C) The peak values of vagal discharge in 30 s increased significantly (p < 0.05). Please click here to view a larger version of this figure.
Supplementary Table 1: The mean heart rate of the rats during the 30 s before and after the 20-min EA session in different groups. *In group E, the mean heart rate showed a significant difference compared to the control, while the other groups showed no significant difference. Please click here to download this table.
Supplementary Table 2: The total number of vagal cluster discharges in rats during the 30 s before and after the 20-min EA session in different groups. *In group E, the total number of vagal cluster discharges of the rats showed a significant difference compared to the control, while the other groups showed no significant difference. Please click here to download this table.
Supplementary Table 3: The peak value of vagal cluster discharge (µV) of the rats during the 30 s before and after the 20-min EA session in different groups. *In group E, the peak values of vagal cluster discharge showed a significant difference compared to the control, while the other groups showed no significant difference. Please click here to download this table.
Discussion
In this experiment, we observed the effects of the different ways of connecting the positive and negative electrodes, including the heart rate, vagal nerve activity frequency, and discharge amplitude. The results showed that when the positive and negative poles were connected to both forelimbs of the body, it affected the emission of bioelectricity. When the positive and negative poles were connected to the same side of the body, the closer the distance between the two needles, the less the effect on bioelectricity. The greater the current, the greater the effect on bioelectricity. So, we recommend the connection mode on the same acupoint, which would not change the bioelectricity.
Generally, the electric field increased with increasing distance between the positive and negative poles of the EA due to body resistance1. It was unclear whether the effect of an electric field would affect the operation of the meridians and the function of the acupoint. To prove this, a more rigorous experimental design is needed. During EA stimulation, both the artifact from the electrodrill stimulation signal and the electrical signals from the tissue are simultaneously captured by the recording device. Consequently, the recorded frequency reflects a synthesis of these two signals, leading to distortion in the recordings. As a result, some researchers discontinue recording during electroacupuncture sessions, which prevents them from observing changes in tissue discharge throughout this process9. In our experiment, even if the current was very low in the connection mode of the contralateral same points and the ipsilateral fore and hind limbs, the frequency of the EA would directly affect the ECG recording and nerve discharge recording. It was called an EA artifact in studies of EA10. Some studies showed the artifacts of EA and have therefore modified some instruments to eliminate the artifacts of EA11. During the experiment, we took many measures to eliminate the stimulus artifacts, such as connecting the ground wire and wrapping the metal device with tin foil. We found that the ECG and nerve discharge in the one acupoint group (Group A) were not affected throughout the experiment. In contrast, in other groups, the stimulation frequency of the EA was superimposed with the measured data. This finding provides a simple and effective solution for studies that need to record data during EA intervention.
The results showed that the ECG and vagal discharge were affected by connecting the fore and hind limbs, both hind limbs and both forelimbs. However, it is difficult to identify whether this effect is good or bad for the body. It is generally believed that the current generated by connecting the forelimbs to the bilateral acupoint passes directly through the heart. Long-term treatment with this method will have some effect on the heart1. This effect should also be further evaluated according to the resistance level of the body and further proven using the disease model. A previous study12 about EA to enhance the effect of drug abortion found that the connection between bilateral acupoints would not only accelerate the discharge of vesicles but also produce the effect of analgesia and shorten the menstrual time. The effects were weakened in the connection between the Hegu and Sanyinjiao points on one side and did not affect the expulsion rates of the fetal sac. However, because drug abortion is destructive to the human body, it was difficult to evaluate the effects of EA in these connection modes. Another study13 compared the effectiveness of two different connection modes of EA at Jiaji points in the treatment of lumbar disc herniation. The results showed that different connection modes of EA at Jiaji acupoints were effective for lumbar disc herniation, and the long-term efficacy of the cross-spinal contralateral Jiaji point connection method was better than that of the ipsilateral Jiaji point connection method.
This experiment is a preliminary observation based on healthy rats, and during the experiment, it was found that there were great differences among the rats. The heart rate of some rats was irregular, and the ECG waveform was unstable at the beginning. In the observation of nerve discharge, it was found that some rats had weak discharge at the beginning. After long-term exposure to air, it was difficult for the nerve to maintain a stable state, and when the rat breathed, the electrodes touched the muscles, resulting in an unobservable discharge. The data analysis function of the instrument we used was relatively simple. In the future, more detailed experimental designs and more advanced instruments are needed to obtain more objective data.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research was supported by National Key Discipline of High Level Acupuncture and Moxibustion Administration of Traditional Chinese Medicine (Grant number zyyzdxk-2023254).
Materials
| Name | Company | Catalog Number | Comments |
| Accupuncture Needle | Hwato | N/A | |
| BL-420N physiological signal recorder | Techman | LAB-0017-0002-CDTM | |
| Electroacupucture instrument | HANS | 200A | |
| Erythromycin ointment | Shuangji | N/A | |
| Mineral oil | Solarbio | 8012-95-1 | |
| Thermostatic rat plate | Techman | JR-30 |
References
- Liu, L., Dai, S. . Clinical Electroacupuncture Therapy. , (2011).
- Han, J. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 26 (1), 17-22 (2003).
- Yang, Z. . Acupuncture and Moxibustion. , (1996).
- Zhang, Z., et al. Electroacupuncture regulates inflammatory cytokines by activating the vagus nerve to enhance antitumor immunity in mice with breast tumors. Life Sci. 272, 119259 (2021).
- Liu, K., Jiang, J., Lu, S. Effect characteristics and mechanism of acupuncture in autonomic nerve regulation. Zhen Ci Yan Jiu. 46 (4), 335-341 (2021).
- Komegae, E. N., et al. Vagal afferent activation suppresses systemic inflammation via the splanchnic anti-inflammatory pathway. Brain Behav Immun. 73, 441 (2008).
- Lu, M., et al. Electroacupuncture at ST36 modulates gastric motility via vagovagal and sympathetic reflexes in rats. World J Gastroenterol. 25 (19), 49-60 (2019).
- Jiang, H., et al. Electroacupuncture pretreatment at Zusanli (ST36) ameliorates hepatic ischemia/reperfusion injury in mice by reducing oxidative stress via activating vagus nerve-dependent Nrf2 pathway. J Inflamm Res. 16, 1595-1610 (2023).
- Ye, Z. A straightforward device for the removal of electroacupuncture artifacts. Journal of the First Affiliated Hospital of Shanghai. 6, 474-475 (1981).
- Huo, R., et al. Responses of primary afferent fibers to acupuncture-like peripheral stimulation at different frequencies: Characterization by single-unit recording in rats. Neurosci Bull. 36 (8), 907-918 (2020).
- Ye, X., et al. Effect of electroacupuncture at "Zusanli" (ST36) on vagal electrical activity in the rat. Acupuncture Research. 5, 290-293 (2006).
- Zhu, J., et al. Clinical observation of electroacupuncture enhancing drug abortion. Chinese Acupuncture and Moxibustion. 7, 389-391 (2000).
- Chen, J., Ni, M., Yin, J. Electroacupuncture treatments for gut motility disorders. Neurogastroenterol. 30 (7), e13393 (2008).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved