Method Article
Rapid Detection of Fecal Antigen of Helicobacter pylori Infection Based on Double Antibody Sandwich Detection Technology
In This Article
Summary
In this study, a sandwich detection technique based on double antibodies was developed for rapid detection of fecal antigens infected with Helicobacter pylori.
Abstract
Helicobacter pylori can be parasitic in gastric mucosa, which can cause a series of gastrointestinal diseases after infection and is closely related to gastritis, gastric ulcer, and gastric cancer. The high prevalence of H. pylori infection in regions with poor medical infrastructure and inadequate sanitation areas remains a significant public health concern. Consequently, the development of rapid, simple, and cost-effective screening methods for H. pylori detection in resource-limited settings is of paramount importance.
In this study, we conducted a community-based screening in Shitan Village, Guangdong Province, a remote and underdeveloped area characterized by a permanent population of approximately 300 residents with generally low educational attainment. We employed a double antibody sandwich assay for the detection of H. pylori fecal antigen, a method chosen for its potential applicability in low-resource settings. A total of 261 participants from the village were enrolled, and their fecal samples were analyzed using this technique. For comparative validation, the same samples were subjected to quantitative polymerase chain reaction (qPCR) analysis. Compared with qPCR results, the sensitivity and specificity of the detection of H. pylori antigen in feces were 60.24% and 80.08%. The results demonstrated a strong agreement between the fecal antigen detection method and qPCR (Kappa = 0.630). This study systematically elucidated the principles, procedures, methodologies, and clinical applications of fecal antigen detection for H. pylori infection, aiming to explore latex-based double antibody sandwich technology and establish novel strategies and practical guidelines for its auxiliary diagnosis.
Introduction
Helicobacter pylori (H. pylori, Hp) is a microaerophilic, spiral-shaped, gram-negative bacterium1. Due to its ability to colonize and chronically infect the human stomach2, this pathogen has been identified as a major etiological factor in the development of gastric adenocarcinoma3,4. In 2015, ~4.4 billion people in the world suffered from H. pylori infection5. The prevalence of H. pylori infection exhibits considerable geographic variation, with developing countries experiencing significantly higher rates compared to developed nations. Notably, in low-income countries and among certain vulnerable populations, the infection rate can reach as high as 75%6.
Although traditional detection methods such as gastroscopy are accurate, their application in large-scale screening and routine follow-up is limited due to their invasiveness, high cost, and low patient acceptance7. Therefore, the search for non-invasive, simple, and rapid diagnostic methods has become an important direction of modern clinical medicine research. The main purpose of a non-invasive diagnosis of H. pylori is to accurately, safely, and conveniently detect whether the patient is infected with H. pylori without endoscopic examination. The urea breath test (UBT), H. pylori fecal antigen test (FAT)8, and serological testing9 are popular noninvasive techniques.
Among these, the UBT is the least intrusive and most accurate procedure available10. The sensitivity and specificity of UBT are higher than 95%11. Among the available non-invasive diagnostic methods, the UBT has been shown to be the most accurate and reliable, as seen in a validation study conducted in Iraq, the UBT may be recommended as the first choice due to its higher performance compared to other methods12. However, UBT also has its drawbacks, such as its high cost and need for mass-spectrometric analysis, which limits the application in remote or resource-limited settings13.
The development of a rapid, non-invasive, and cost-effective diagnostic method for detecting H. pylori infection is critically needed, particularly in resource-limited regions. In this study, we developed a rapid detection technology for H. pylori fecal antigen based on double antibody sandwich detection technology, which can detect H. pylori antigen in fecal samples quickly, effectively, and at low cost. This technology offers significant advantages, including affordability, non-invasiveness, user-friendliness, and adaptability to remote areas. We hypothesize that the double antibody sandwich method for detecting H. pylori fecal antigen will demonstrate sensitivity and specificity comparable to qPCR, positioning it as a viable tool for non-invasive clinical diagnosis.
We comprehensively detailed the principles, procedures, methodological strengths, and clinical applicability of fecal antigen detection for H. pylori infection. To validate its practicality, we conducted an epidemiological investigation and screening in Shitan Village, Qingyuan City, Guangdong Province, China, which is a remote and underdeveloped area with a permanent population of approximately 400 and limited educational attainment. Our results demonstrate that the rapid detection technology of H. pylori antigen in feces designed here can be completed within 20 min. It is highlighted that the rapid detection of H. pylori antigen in feces is a potential and promising tool for rapid and reliable diagnosis of H. pylori infection in remote and backward areas.
Protocol
This cross-sectional study has been approved by the Ethics Committee of Guangdong Provincial People's Hospital (Approval Number: KY2024-445-01), and the personal data of all study subjects was strictly confidential during the study. All participants signed written informed consent before the experiments. The residents of Shitan village in Qingyuan City, Guangdong Province, in 2024 were selected as the research subjects, and there were no restrictions on age and sex. The double antibody sandwich assay (a qualitative experiment) described below was performed by professional medical and technical personnel according to the instructions. The entire permanent population of the village was selected for this study, regardless of whether they were healthy or had symptoms or any existing diseases related to gastrointestinal diseases.
1. Patient selection
- Exclude patients if they have used proton pump inhibitors or bismuth, H2 receptor blockers, or antibiotics in the previous 1 month.
- Ask all participants to fill in the questionnaire anonymously. The contents of the questionnaire include five questions: Do you eat three meals regularly? Do you drink alcohol? Are you aware of H. pylori? Do you think it is necessary to carry out H. pylori inspection? Do you understand the adverse consequences of H. pylori infection?
2. Procedure for fecal H. pylori infection antigen detection
NOTE: The algorithm for H. pylori stool antigen testing was designed to ensure the accuracy and reliability of the results, with consideration for simplicity and hygiene. The whole process is divided into five steps: sample collection, sample processing, sample storage, detection, and result interpretation. The working flow chart and schematic diagram of sample detection are shown in Figure 1 and Figure 2.
- Sample collection
NOTE: H. pylori antigen-specific containers should be used for sample collection. The samples should not be mixed with water, urine, disinfectants, and sewage.- After the completion of defecation, remove the top lid of the special container to take out the sampling rod.
- Insert the sampling rod continuously into five different positions of the stool for sampling, making sure that the threaded end of the sampling rod is completely inserted into the stool.
- Insert the sampling rod back into the reagent tube after the sampling is completed; the sample volume is ~5-50 mg in total.
- Sample processing
- Shake the reagent tube from side to side for ~10 s to thoroughly mix the stool sample in the diluent (main ingredients: ethylenediamine tetraacetic acid tetrasodium hydrate 0.018 g/mL and sodium chloride 0.01 g/mL).
- Sample storage
- Test the samples as soon as possible after sampling; store them at room temperature for no more than 6 h and under refrigerated conditions (no more than 72 h at 2-8 °C and no more than 6 months frozen at -25 °C to -15 °C) if necessary to slow down antigen degradation and microbial activity.
NOTE: Samples can be repeatedly frozen and thawed up to 3x, and both refrigerated and frozen samples should be returned to room temperature before testing.
- Test the samples as soon as possible after sampling; store them at room temperature for no more than 6 h and under refrigerated conditions (no more than 72 h at 2-8 °C and no more than 6 months frozen at -25 °C to -15 °C) if necessary to slow down antigen degradation and microbial activity.
- Sample detection
- Open the white cover on the reagent tube cover and keep the reagent tube erect.
- Press down the lid to the lowest point so that the sample flows on the test card, passing through the detection area (T) and quality control area (C) coated with the mouse anti-Hp antibody.
- Interpretation of results
- Wait for 10-20 min so that any H. pylori antigen in the sample, if bound to the antibody, forms a macroscopic chromogenic reaction in the detection area. This usually appears as a red or purple line that contrasts with the line in the quality control area.
- The presence or absence of the color reaction and the depth of the color are related to the concentration of H. pylori antigen in the sample. Compare the color cards to quickly and accurately interpret the results (see Figure 3).
3. DNA extraction
- Place an appropriate amount of feces into a test tube, add PBS solution, and mix thoroughly by shaking. Centrifuge the mixture at 12,000 × g for 5-10 min, then carefully collect 200 µL of the supernatant for further extraction.
- Based on the number of samples, prepare an equal number of 1.5 mL centrifuge tubes. To each tube, sequentially add 20 µL of proteinase K (20 mg/mL), 10 µL of magnetic beads, 200 µL of the sample supernatant, and 200 µL of lysis buffer A. Mix thoroughly by inverting the tubes, then incubate in a metal bath at 55 °C for 10 min.
- After the pyrolysis is completed, place the centrifuge tube on the magnetic frame, let it stand, and allow adsorption to take place for 1 min on the beads. After the liquid in the tube is completely clarified, discard the supernatant and try to avoid disturbing the magnetic beads.
- Remove the tubes from the magnetic rack and place them on a 1.5 mL centrifuge tube rack. Using a pipette, add 500 µL of wash buffer E to each tube, mix thoroughly for 1 min, then return the tubes to the magnetic rack. Allow the tubes to stand for 1 min, discard the supernatant after clarification, and avoid aspirating the magnetic beads.
- Repeat the washing process by adding 500 µL of wash buffer W2 to each tube. Mix thoroughly for 1 min, place the tubes on the magnetic rack, and let them stand for 1 min. Discard the supernatant carefully after the solution clarifies, ensuring the magnetic beads remain undisturbed.
- Add 100 µL of eluent, mix, and incubate at 55 °C for 5 min.
- Place the tubes on the magnetic rack and let them stand for 2 min. Once the solution clarifies, carefully transfer the eluted nucleic acid into a new centrifuge tube, avoiding aspiration of the magnetic beads.
- Evaluate the purity of the extracted DNA by measuring the absorbance at 260 nm (A260) and 280 nm (A280). Calculate the A260/A280 ratio, which should ideally be greater than 1.8 for high-purity DNA.
4. qPCR for the detection of H. pylori and resistance to antibiotics
NOTE: We performed qPCR for the detection of H. pylori infection by amplifying the ureA gene. All the negative quality control products are sterile, purified water. The positive quality control product in the H. pylori nucleic acid detection kit is inactivated H. pylori standard beads (ATCC 43504). A CT ≤ 30 with a typical S-shaped curve is considered positive.
- According to the number of samples to be tested, take the H. pylori freeze-dried preparation out of the kit, and quickly spin it to keep the freeze-dried powder at the bottom of the tube.
- Carefully open the lid of the freeze-dried preparation; take the nucleic acid of the sample to be tested; add 25 µL the sample nucleic acid into the freeze-dried preparation; and cover the tube tightly.
- Shake and mix the PCR reagent evenly for 8-10 s, and then quickly spin it for 3-5 s.
- For each sample on a 32-well plate, prepare 25 µL of the PCR reaction mixture (freeze-dried powder [containing the Taq enzyme (5 U/µL), deoxyribonucleoside triphosphate (2.5 mmol/L), UNG enzyme (2 U/µL) , ureA forward (5'-ACATTGCGAGCGGGACAG-3') and reverse (5'-CGCCCAATCTCACTTTATCG-3') primers (40 µmol/L)]), and 25 µL (2.5 µg) of the extracted DNA.
- Run the 32-well plate qPCR board on the qPCR machine.Program the thermal cycler: 42 °C and 95 °C (both for one cycle) for 2 min each; 95 °C for 10 s and 65 °C for 45 s (two steps are one cycle) for 10x; 95 °C for 10 s and at 58 °C for 45 s (two steps are one cycle) for 35 cycles.
- Analyze the data using specific software for qPCR and let the instrument automatically select baseline thresholds.
5. Statistical analysis
- Use the chi-square test to analyze the experimental data to evaluate the consistency between this method and the qPCR results and compare the positive rate of this technology screening with the positive rate data of H. pylori infection on a wider scale (e.g., nationwide). Consider differences statistically significant at P < 0.05.
Results
Questionnaire survey
A total of 261 participants were enrolled in the study, comprising 144 females and 117 males, with ages ranging from 4 to 99 years. The average age of the subjects was 48.30 ± 17.61 years. There were 17 minors (0-17 years old), 202 adults (18-64 years old), and 42 elderly participants (>64 years old). The questionnaire results are shown in Table 1. The majority (90.8%) of the subjects thought it was necessary to carry out H. pylori screening.
Screening results of fecal H. pylori antigen detection in this village
Among the 261 participants tested for fecal H. pylori antigen, 52 (19.92%) were positive, while 209 were negative. Of the 52 positive cases, 32 were female, representing 22.22% of all female participants, and 20 were male, accounting for 17.09% of all male participants. When stratified by age, the positivity rates were as follows: minors (0-17 years) had 2 positive cases (11.76%), adults (18-64 years) had 41 positive cases (20.30%), and the elderly (>64 years) had 9 positive cases (21.43%).
Comparison of consistency between fecal H. pylori antigen and qPCR results
In this study, two samples with different qPCR test results were selected to characterize the reliability of the experimental protocol (Figure 4). Among the fecal H. pylori antigen results of 261 subjects, 52 were positive and 209 were negative. Among the results of qPCR with the feces of 261 subjects, 83 were positive and 178 were negative (Table 2). The sensitivity and specificity of fecal H. pylori antigen detection were 60.24% and 80.08%, respectively. The positive predictive value (PPV) of this method was 96.15%, and the negative predictive value (NPV) was 84.21%. The Kappa consistency test indicates that the diagnosis results of the two methods are consistent (Kappa = 0.630, P < 0.05).
Comparison of screening positive rate between this natural village and the whole country
The positive rate was 19.92%, which was slightly lower than the national positive rate of 42.8%14 (Table 3). According to the chi-square test (P < 0.01), the difference in the positive rates was statistically significant.

Figure 1: Flow chart of sample detection. Please click here to view a larger version of this figure.
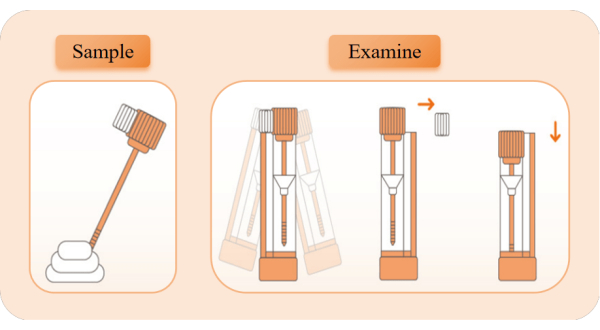
Figure 2: The schematic diagram of sample collection and detection. Take out the detection device, tear open the aluminum foil bag, and take out the reagent tube. Pull off the orange tube cover and use the sampling stick to take samples 5x from different positions of feces. Insert the sampling rod back into the reagent tube and shake it left and right for 10 s to fully mix. Break the white limit block, keep the reagent tube upright, and press the tube cover to the lowest position to start timing. Observe the appearance of red bands. Please click here to view a larger version of this figure.

Figure 3: Interpretation of antigen results. (A) Sampler. (B,E) Negative (-): A negative result indicates that there is no H. pylori antigen detected in the sample, suggesting that the risk of H. pylori infection is low. A negative result cannot completely rule out the possibility of infection. (C,F) Positive (+): A positive result means that H. pylori antigen is detected in the sample, H. pylori infection is suspected. (D) No effect: Invalid results may be a problem with the sample collection or handling process, or the reagent has deteriorated and damaged, and the sample should be collected again or the operation steps should be repeated. Please click here to view a larger version of this figure.
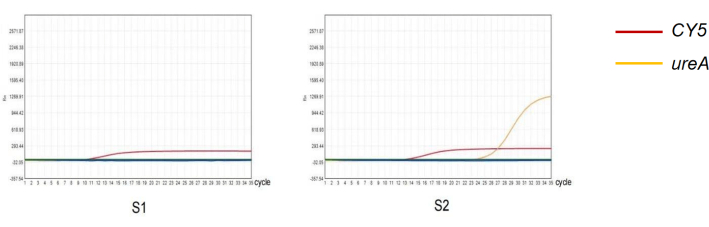
Figure 4: Detection of H. pylori in feces by qPCR method. (S1) Negative result of quantitative PCR amplification of H. pylori infection. (S2) Positive result of quantitative PCR amplification of H. pylori infection. Please click here to view a larger version of this figure.
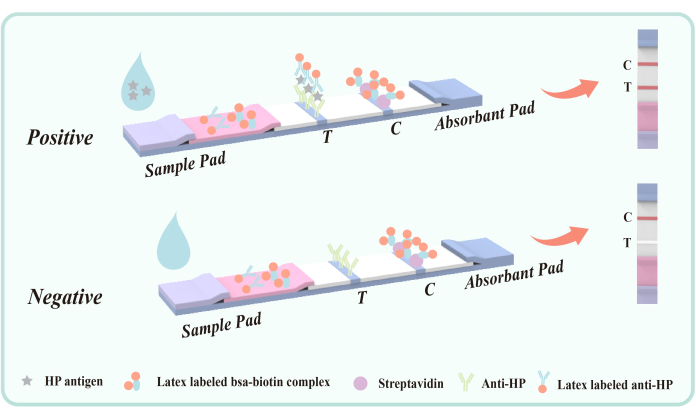
Figure 5: Schematic diagram of double antibody sandwich method for detecting fecal antigen infected with H. pylori. Mouse anti-Hp antibody (VacA epitope) was fixed in the detection area (T) on the membrane in advance. The quality control area (C) fixes goat anti-mouse IgG and streptavidin in advance. The polyester film was coated with latex labeled mouse anti-Hp antibody and latex-BSA- biotin conjugate. Please click here to view a larger version of this figure.
| Whether to eat regularly for three meals? | Whether to drink alcohol? | Do you know H. pylori? | Whether it is necessary to carry out H. pylori inspection? | Do you understand the adverse consequences of H. pylori infection? | |
| Yes (rate) | 236 (90.4%) | 59 (22.6%) | 117 (44.8%) | 237 (90.8%) | 104 (39.8%) |
| No (rate) | 25 (9.6%) | 202 (77.4%) | 144 (55.2%) | 24 (9.2%) | 157 (60.2%) |
Table 1: The questionnaire results.
| H. pylori antigen | Total | |||
| Positive (+) | Negative (-) | |||
| qPCR | Positive (+) | 50 | 33 | 83 |
| Negative (-) | 2 | 176 | 178 | |
| Total | 52 | 209 | 261 | |
Table 2: Diagnostic results of two techniques.
| Positive | Negative | Positive rate | Total | |
| Shitan village | 52 | 209 | 19.92% | 261 |
| China | 314423 | 449404 | 42.80% | 763827 |
Table 3: Comparison of H. pylori screening positivity rates between Shitan Village and the national average.
Discussion
H. pylori represents one of the most widespread bacterial infections all over the world15. The questionnaire results revealed that 55.2% of the population lacked awareness of H. pylori, while 90.8% believed that H. pylori screening is necessary. These findings underscore the importance of implementing H. pylori screening programs in economically disadvantaged and remote areas16. Although the urea breath test (UBT) is a common diagnostic method, its high cost and unsuitability for children and pregnant women limit its applicability17,18. Serum H. pylori antibody test can detect antibodies in a patient's serum19. However, this test is not suitable for posttreatment analysis since the antibodies remain long after the bacteria have been cleared20. In contrast, stool antigen testing offers a non-invasive approach that eliminates the need for gastric cavity access, requiring only stool samples for analysis. This method significantly reduces patient discomfort and potential risks, making it more convenient for both screening and follow-up. Additionally, its non-invasive nature makes it more acceptable to populations in remote and underdeveloped regions.
The prevalence of H. pylori-positive patients in our study population is 19.92%, which was slightly lower than the national positive rate of 42.8%14. According to the chi-square test (P < 0.01), there was statistical significance between them. The reason for this difference may be the infection rate of H. pylori in urban populations in seven geographical regions of China has obvious regional differentiation21. The infection rate is highest in East China, higher in Northeast China, North China, and Northwest China, and lower in South China and Southwest China21. The area we screened belongs to South China, and the infection rate is lower than the national level. On the other hand, the nonstandard operation in the sampling process of individual fecal samples and the long time to send for testing may also be the reasons for this difference. Although PCR and its derivatives are extensively employed for detecting various pathogens, including H. pylori, their utility is limited by the complexity and cost of thermocycler equipment and the need for specialized operators22,23. In this study, compared with qPCR results, the sensitivity and specificity of the detection of H. pylori antigen in feces were 60.24% and 80.08%. The consistency of H. pylori antigen in feces and qPCR results was compared, and the results suggested that they were moderately consistent (Kappa = 0.630, P < 0.05).The reasons for its unsatisfactory sensitivity may be influenced by irregular sample collection, sample storage temperature, and delivery time.
The double-antibody sandwich method for the detection of H. pylori infection fecal antigen is an efficient and specific immunological detection technology. The core of this method is to take advantage of the specific binding ability of antibodies and ensure accurate identification and quantification of target antigens through an ingenious detection system. Specifically, this approach involves two key steps: antigen capture and antibody detection. The schematic diagram of this technology is shown in Figure 5.
The mouse anti-Hp antibody coated on the polyester membrane detection region (T) targets the H. pylori VacA epitope to achieve specific capture of H. pylori antigen in feces. VacA antigen is an important exotoxin of H. pylori, closely associated with infection. Antibodies targeting VacA can effectively identify and bind to the antigen, forming an antigen-antibody complex. Therefore, this method is not easy to cross-react with other gastrointestinal diseases or diseases.
During the test, the liquid sample enters the detection tank and migrates upward through capillary action. If the sample contains H. pylori antigen, it first forms an antigen-antibody complex with the latex-labeled mouse anti-H. pylori antibody coated on the polyester film. As the complex flows through the detection zone (T), it is captured by the immobilized mouse anti-H. pylori antibody, resulting in a visible red band in the detection zone (T), which indicates a positive result. If no H. pylori antigen is present in the sample, no double antibody sandwich complex forms in the detection zone (T), and consequently no red band appears, indicating a negative result. Regardless of the presence or absence of H. pylori antigen, the latex-BSA-biotin conjugate will bind to the streptavidin immobilized on the membrane during chromatography, producing a red band in the quality control zone (C). This red band serves both as an indicator of proper chromatographic process and as the internal control standard for the reagent.
The double-antibody sandwich method realizes the efficient capture and qualitative detection of H. pylori antigen in feces by carefully designed antibodies and markers. Its simplicity, rapidity, and high specificity provide reliable technical support for the assistant diagnosis of H. pylori infection. The widespread application of this technology not only reduces medical costs and enhances screening efficiency but also provides patients with a more comfortable and convenient testing experience. This advancement holds significant importance for the global prevention and control of H. pylori infection.
In addition, the hygiene of stool antigen testing is a critical consideration. This method requires fresh fecal samples, which can be either formed or unformed, with no specific restrictions on collection time. During sample collection, it is essential to avoid contamination with water, urine, disinfectants, or sewage. Fecal samples should be collected in clean, dry containers free of preservatives and detergents. If storage is necessary, samples can be kept at room temperature for no more than 6 hours or at 2-8 °C for up to 72 h. Refrigerated samples must be brought back to room temperature prior to testing to ensure accurate results. High time efficiency is also a major feature of stool H. pylori infection antigen detection. The latex-based double antibody sandwich assay can provide results in 10-20 min compared to traditional methods that require waiting for laboratory results, which is important for the recognition of acute infection and early treatment decisions in remote and backward areas.
In summary, stool H. pylori antigen detection offers numerous methodological advantages, including non-invasiveness, simplicity, hygiene, high efficiency, and broad applicability. These features make this method a valuable tool for clinical practice and provide innovative insights for the future development of diagnostic technologies. In underdeveloped regions with limited sanitary conditions, this approach is poised to play a pivotal role in the diagnosis and management of H. pylori infection.
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgements
None
Materials
| Name | Company | Catalog Number | Comments |
| DNA extraction kit | Jiangsu mole biotechnology co., ltd | 20230223 | None |
| H. pylori fecal antigen detection kit | Hangzhou nuohui healthy technology co., ltd | 20213401126 | None |
| H. pylori nucleic acid detection kit | Jiangsu mole biotechnology co., ltd | 20230226 | None |
| Real-time fluorescence quantitative PCR | Shanghai Hongshi medical treatment technology co., ltd | 20183221659 | None |
| Statistical Product and Service Solutions | IBM company | None | None |
References
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved